Organ and Tissue Donation Following Medical Assistance in Dying: Guidance for Policy Meeting Report
Executive Summary
Background
Following the decriminalization of medical assistance in dying (MAiD) in 2015, Canadian Blood Services in collaboration with the Canadian Critical Care Society, the Canadian Society of Transplantation and the Canadian Association of Critical Care Nurses developed Organ and Tissue Donation for Medical Assistance in Dying and Other Conscious Competent Patients: Expert Guidance for Policy published in 2019.
On March 17, 2021, Bill C-7 was adopted changing legislation for Canada in the areas of eligibility, safeguards, consent and monitoring regime. These changes to the MAiD legislation required community consideration for their impact on organ and/or tissue donation processes and offered an opportunity to improve and update the current guidance for policy.
Meeting Objectives and Process
In June 2021 and April 2022, Canadian Blood Services assembled an interdisciplinary group of experts to examine the changes to legislation and their impact on organ and/or tissue donation. A planning committee was established to develop the meeting agendas and to prepare background materials for review by participants in advance of the meetings. Experts were invited from backgrounds that would intersect with the care of an organ donor who was approved for MAiD: critical care (physicians and nurses), donation physicians, experts in bioethics and law, MAiD assessors and providers, organ donation organizations (ODOs), abdominal transplant surgeons, and organ donation and transplant researchers. Patients who are considering and/or have consented to organ and/or tissue donation following MAiD as well as family members who supported loved ones through organ and/or tissue donation following MAiD were also included. Formal representation from professional societies included: Canadian Critical Care Society, Canadian Association of Critical Care Nurses, Canadian Society of Transplantation, Canadian Donation and Transplantation Research Program, Canadian Blood Services and the Canadian Association of MAiD Assessors and Providers.
The objectives of these meetings were:
- Examine the potential impact of loss of capacity and waiver of final consent on consent for organ and/or tissue donation for MAiD patients whose natural death is reasonably foreseeable (Track One MAiD patients).
- Determine when to approach MAiD patients whose natural death is not reasonably foreseeable (Track Two MAiD patients) for formal consent to organ and/or tissue donation.
- Examine emerging questions, such as directed donation following MAiD and organ and/or tissue donation following MAiD at home.
- Discuss national data collection, professional education, knowledge gaps and research opportunities to inform future strategy development.
Before the meetings, participants reviewed information that would assist in discussions and evaluation of proposed policy changes. During the meetings, attendees heard from national and international experts. In addition, discussions were held in small groups, panels and in plenary.
Meeting Outcomes
Based on the information presented and discussions at the meetings:
- Under the circumstances where a Track One MAiD Patient has provided first-person consent for MAiD and first-person consent for organ/tissue donation, but loses the capacity to reaffirm consent prior to death, consensus was provided in support of upholding first-person consent for organ and/or tissue donation and coordinating next steps with the substitute decision-maker (SDM):
-
Image
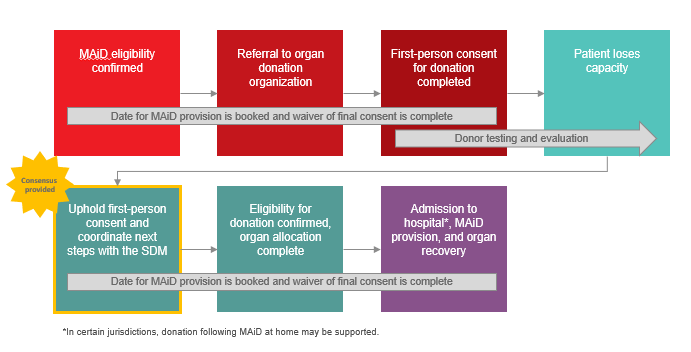
-
- Under the circumstances where a Track One MAiD Patient has provided first-person consent for MAiD but loses capacity prior to the organ/tissue donation consent discussion, consensus was provided in support of approaching the SDM to 1) reaffirm consent for registered donors and those in a jurisdiction with opt-out legislation, or 2) obtain consent from SDM for those without a registered consent decision:
-
Image
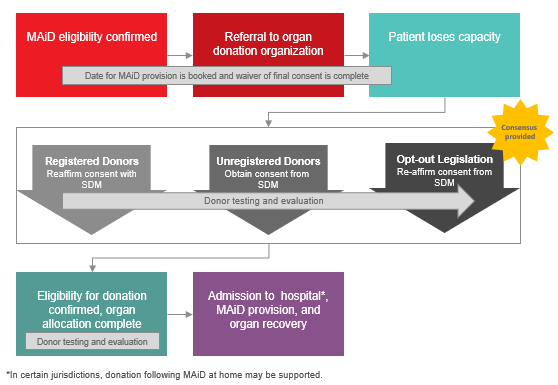
-
- Consensus that ODOs and transplantation programs should implement a directed deceased donation policy for patients pursing MAiD, in alignment with living directed donation practices and principles. The following principles were thought to be appropriate for guiding deceased directed donation following MAiD:
- General
- Living donation prior to death from patients considering MAiD or withdrawal of life-sustaining measures (WLSM) should be neither offered nor encouraged.
- Requests for deceased directed donation should be considered on a case-by-case basis.
- Confirmation of eligibility for MAiD must happen before approach and consent for organ and/or tissue donation, and eligibility for organ and/or tissue donation must be established before any discussion of directed deceased donation.
- Patient Request for Directed Donation
- A patient’s request for directed deceased donation following MAiD must be made voluntarily.
- Directed donation should not proceed if there is indication of monetary exchange or similar valuable consideration and/or coercion are involved in the decision to pursue directed donation.
- The intended recipient in a directed deceased donation case should be a family member or ‘close friend’, an individual with whom the donor or donor’s family has had a long-standing emotional relationship. This practice is consistent with living organ donation, in which the specified recipient is most often closely related to the donor.
- Consent
- Consent for directed donation following MAiD will not be considered in cases where a patient and/or the substitute decision-maker places conditions on the member of a group, class, or organization to whom should be in receipt of the organ if the intended donation cannot be realized (e.g., an individual will not donate to patients with history of drug/alcohol abuse or to certain racial groups).
- In line with the current Canadian guidance, the patient should be informed and understand that they may withdraw consent for MAiD or donation at any time, and that withdrawal of consent for donation does not affect their consent for, or access to, MAiD or WLSM.
- Intended Recipient Eligibility
- The intended recipient must be on the current transplant waiting list, or meet the following criteria:
- meets the transplant program’s listing criteria,
- has completed the work-up for organ transplantation, and/or
- the need for a transplant is clinically indicated and evaluation for transplant has been completed for the intended recipient, as determined and confirmed by the appropriate transplant program.
- Although directed donation following MAiD may lead to better compatibility between the donor and the intended recipient compared to another unrelated recipient, the recipient in the greatest need of the organ will be prioritized.
- Transplantation will only proceed if the donor organ is medically compatible to the intended recipient.
- The intended recipient should be informed and understand that they may withdraw consent for transplantation at any time, and that withdrawal of consent for transplantation does not affect their position on the waitlist.
- The intended recipient must be on the current transplant waiting list, or meet the following criteria:
- General
- Consensus was provided in support of referring Track Two MAiD Patients to ODOs prior to confirmation of MAiD eligibility if this was a patient initiated inquiry for the purposes of information sharing. Otherwise, there was consensus for approaching Track Two MAiD Patients for first-person consent to organ and/or tissue donation after MAiD eligibility has been confirmed. Approach and consent for donation may occur during, or after, the mandatory 90-day assessment period which has been established as a safeguard in the process.
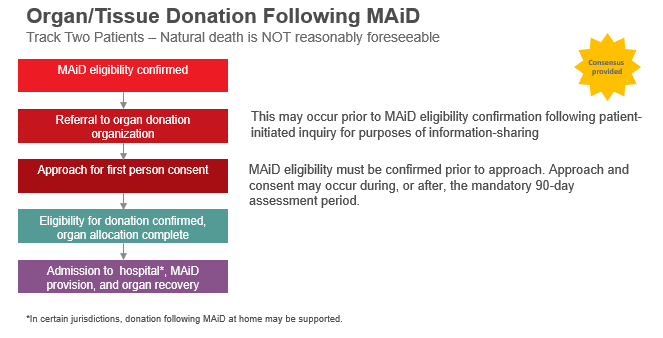
- General agreement from the majority of meeting participants (88%) that organ and/or tissue donation following MAiD at home should be offered in Canada. Despite this, there was significant uncertainty about logistics and feasibility in most jurisdictions.
- Consensus that collecting and reporting data specific to organ and/or tissue donation following MAiD is an important aspect of performance measurement and required for continued improvement and future strategy development. Data elements to be collected include:
- Number of MAiD patients referred for organ and/or tissue donation
- Number of referred MAiD patients eligible for organ and/or tissue donation
- Number of referred MAiD patients approached for organ and/or tissue donation
- Number of MAiD patients consented to organ and/or tissue donation
- Number of utilized organs from MAiD donors
- Number of utilized tissue donations from MAiD donors
- Average number of organs transplanted per MAiD donor
- Consensus that health-care professionals involved in organ and/or tissue donation following MAiD require specialized education and training.
- Identification of important knowledge gaps and research opportunities.
Next Steps
Using the information generated during these forums, a journal publication to inform the Canadian stakeholder community of the work and guide future efforts will be developed in addition to this comprehensive meeting report.
Based on the outcomes in this report, guidance for policy in organ and/or tissue donation following MAiD at home may be feasible. However, further work is necessary to assess the potential for a medical, legal and ethical framework—including feasibility and logistics—for organ and/or tissue donation following MAiD at home in the Canadian context.
Canadian Blood Services will work with community stakeholders to develop a strategy to improve organ and/or tissue donation following MAiD practices (including directed donation), data collection and reporting and improved education and training for those involved in these complex cases. Further, Canadian Blood Services will share the knowledge gaps and research opportunities with clinician scientists in the field with the hopes of having some of these gaps addressed.
Acknowledgements
We would especially like to thank Nichole Riese, Sandra Wadsworth, Randy Tresidder and Donna Wonnick—our donor family and patient partners—whose experiences were openly shared during their full participation throughout the meetings. Their perspectives kept the meetings focused on the overarching purpose that drove this initiative: providing high-quality end-of-life care and organ and/or tissue donation options for dying patients while protecting their interests, supporting grieving families and improving transplant opportunities for patients on waitlists.
Disclosures
These meetings were supported financially by Canadian Blood Services through a contribution from Health Canada in support of developing leading practices.
Canadian Blood Services receives funding from the provincial and territorial Ministries of Health and the federal government through Health Canada. The views expressed herein do not necessarily represent the views of the federal, provincial or territorial governments. Canadian Blood Services is a national, not-for-profit charitable organization that manages the supply of blood and blood products in all provinces and territories in Canada (with the exception of Québec) and oversees the Canadian Blood Services Stem Cell Registry. In 2008, Canadian Blood Services became responsible for national activities related to organ and tissue donation and transplantation (OTDT), which includes national system development and operation of interprovincial organ sharing programs. Canadian Blood Services is not responsible for the management or funding of any Canadian organ donation organization or transplant program.
The complete forum report .pdf is available upon request in English and French. Requests for pdf copies can be sent to OTDT@blood.ca