Chapter 10
Transfusion reactions
Summary
This chapter covers various transfusion reactions that can occur with the transfusion of blood components (i.e., red blood cells [RBC], platelets, plasma). For information on transfusion reactions related to plasma protein and related products (PPRP), see the chapters on specific blood products: Chapter 3 on albumin, Chapter 4 on immune globulin products, and Chapter 5 on concentrates for hemostatic disorders and hereditary angioedema. Informed consent is described as a vital part of the transfusion process; for more information see the course, Informed consent for blood transfusion, on the professional education website. Prevention of transfusion reactions through patient assessment, the use of premedications where appropriate, donor management/testing, or component modification, is also explored. However, some reactions are idiosyncratic and may not be preventable.
Overall, the supply of blood components in Canada is safe with very low infectious risks. The risk of transfusion reaction was 1 per 2,600 units of blood components transfused based on the Transfusion Transmitted Injuries Surveillance System (TTISS) report for 2018-2019.1 The focus of this chapter will be on transfusion reactions and not errors and accidents. There are multiple ways to approach understanding transfusion reactions (e.g., acute versus delayed, infectious versus non-infectious); this chapter will provide a systems-based approach.
Hemovigilance is the surveillance of blood transfusions, and it is a key aspect of continuous quality improvement.2 Recognizing, managing, preventing, and reporting transfusion reactions are crucial aspects of maintaining a safe blood supply. Reporting requirements in Canada, excluding Quebec, are described in more detail in Canadian Blood Services’ A Guide to Reporting Adverse Transfusion Reactions.
Informed consent
Informed consent is a necessary part of medical care in which health-care practitioners provide patients with information they need to make voluntary treatment decisions, including those related to the transfusion of blood.3 The key tenets of informed consent to any treatment are: (1) it is voluntary, (2) the patient has decision-making capacity, and (3) the patient has been properly informed.4 Obtaining informed consent involves a discussion of the benefits, risks and alternatives for the proposed treatment. Patients have the right to ask questions, accept or refuse treatment, and subsequently change their minds. In order to seek consent for transfusion, health-care practitioners need to have a general understanding of transfusion reaction risks. For more on informed consent see Chapter 9 on blood administration.
In emergency situations where informed consent cannot be obtained from the patient or a substitute decision maker, physicians are expected to provide treatment if the patient’s life or health is at imminent risk.4 It is imperative that physicians respect any advanced directives or other known wishes of the patient.
Patients may refuse blood transfusion for any reason, including concerns about transfusion reactions, cultural reasons or religious beliefs. In these cases, it is especially important that clinicians maintain a strong rapport with their patients, advocate for them, and document their wishes.
Assessment for acute transfusion reaction risk
Patients should be made aware of the possible risks associated with transfusion of blood components and be carefully assessed before transfusion. Reactions such as transfusion-associated circulatory overload (TACO) or transfusion-associated graft versus host disease (TA-GvHD) can be mitigated (see Table 2). Patients must also be monitored carefully for signs and symptoms of transfusion reactions during and after transfusion (Figure 1). More information on patient monitoring is provided in Chapter 9 on blood administration.
Table 1. Acronyms used to describe transfusion reactions.
| AHTR | Acute hemolytic transfusion reaction |
| ARDS | Acute respiratory distress syndrome |
| BACON | Bacterial contamination |
| DHTR | Delayed hemolytic transfusion reaction |
| FNHTR | Febrile nonhemolytic transfusion reaction |
| TACO | Transfusion-associated circulatory overload |
| TAD | Transfusion-associated dyspnea |
| TA-GvHD | Transfusion-associated graft versus host disease |
| TRAIN | Transfusion-related alloimmune neutropenia |
| TRALI | Transfusion-related acute lung injury |
| PTP | Post-transfusion purpura |
Table 2. Factors to consider prior to transfusion.
| Premedication |
The evidence for premedication is weak and the use of acetaminophen and diphenhydramine has been shown to be ineffective in preventing febrile nonhemolytic transfusion5 reactions (FNHTR) or allergic reactions.6 Premedications should be considered on a case-by-case basis for patients who have experienced a transfusion reaction in the past. |
| Volume status |
Risk factors for TACO include a history of congestive heart failure, coronary artery disease, renal failure, and extremes of age.7, 8 To mitigate volume overload, a slower rate of transfusion and pre-transfusion diuretics can be considered. In stable non-bleeding patients, it is prudent to transfuse one unit at a time then re-assess.9 |
| Modified and phenotype-selected blood components |
Irradiated blood The National Advisory Committee (NAC) on Blood and Blood Products 2018 recommendations outline which patient populations should receive irradiated blood.10 This is discussed in more detail in the section below on transfusion-associated graft versus host disease. More information on irradiated blood components is provided in Chapter 15. Antigen-negative blood Patients with rare blood phenotypes or alloantibodies may require blood that is negative for specific antigens. Washed blood components The goal of washing is to remove plasma from the blood component. Washing can be labour-intensive and can decrease the shelf life of a component so it should be used judiciously. For example, it can be considered in those with previous severe reactions. More information on washed blood components is provided in Chapter 15. |
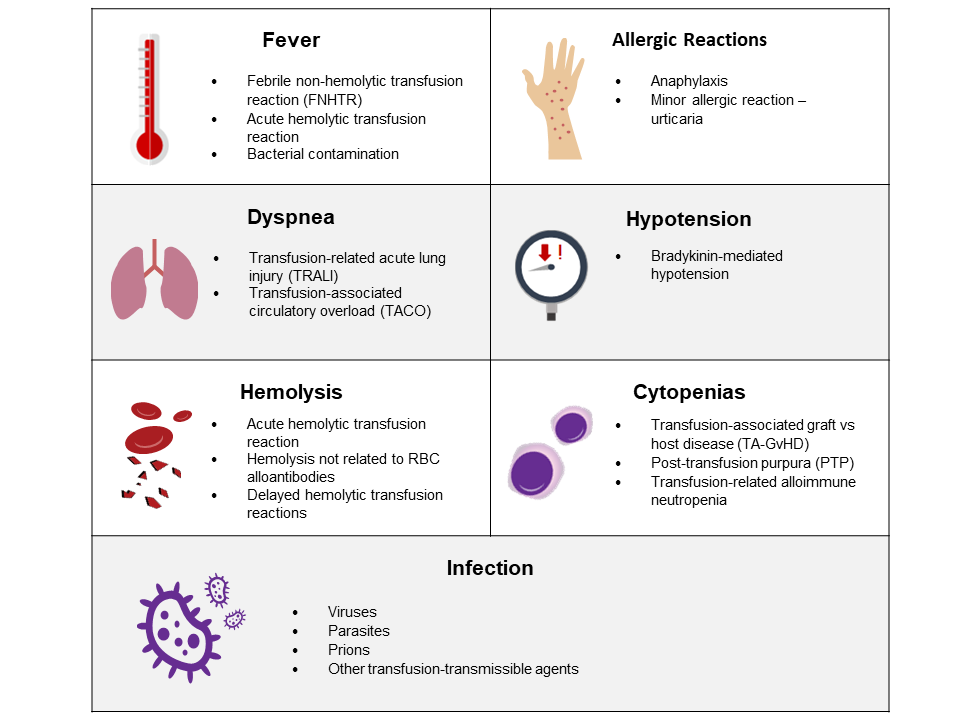
Fever
Fever may be diagnosed in a patient receiving a transfusion when there is a greater than 1°C increase in temperature and/or the temperature is greater than 38°C during or up to 4 hours post-infusion.
The differential diagnosis of fever during a transfusion includes a febrile non-hemolytic transfusion reaction (FNHTR), acute hemolytic transfusion reaction (AHTR), transfusion-related acute lung injury (TRALI), bacterial contamination (see FAQ: Canadian Blood Services platelet bacterial screening), and etiologies that are not related to the transfusion itself (e.g., underlying sepsis).
Febrile non-hemolytic transfusion reaction
FNHTR is one of the most common transfusion reactions with an estimated incidence of 4.6% with platelet transfusion and 0.33% with red blood cell transfusion.6 In Canada, FNHTR is estimated to occur in 2.4 of every 1,000 transfusions.11 FNHTR can also manifest as chills, rigors, or pain.11 It is thought to be the result of inflammatory cytokines or recipient antibodies against donor leukocytes.11 Management is largely supportive and can include acetaminophen. In rare circumstances of severe rigors, meperidine can be considered for symptomatic relief. Prophylactic use of acetaminophen and diphenhydramine is ineffective in decreasing occurrence of FNHTR.12
Acute hemolytic transfusion reaction
AHTR is a rare but potentially devastating transfusion reaction. Symptoms can include fever, chills, hemoglobinuria, pain, dyspnea, hypotension, and oliguria.13 Immune causes of AHTR include ABO-incompatibility, acquired anti-A and anti-B in those who are not group O, and incompatibility due to other blood group systems. The severity of an AHTR can depend on various factors such as the volume of component transfused, characteristics of the alloantibody, and other underlying conditions a patient may have. If an AHTR is suspected, the transfusion must be stopped immediately and the blood bank should be notified.
Laboratory investigations can demonstrate serologic incompatibility (a positive direct antiglobulin test [DAT], incompatibility with repeat crossmatch), evidence of hemolysis (elevated lactate dehydrogenase [LDH], increased bilirubin, low haptoglobin, hemoglobinemia) acute renal impairment and disseminated intravascular coagulation. Supportive management includes close monitoring and hydration.13 Clerical errors are a significant contributor to ABO-incompatible transfusion, therefore strategies for prevention of mistransfusion are crucial. Such preventive strategies may include electronic patient identification,14 judicious use of blood components, a strict specimen labelling policy, and thorough investigation of transfusion errors when they occur.
Bacterial contamination
Bacterial contamination (BACON) is an uncommon risk of transfusion. Approximately 0.09% of platelet pools and 0.04% of apheresis platelet units are found to have confirmed positive results for bacteria at Canadian Blood Services.15 The rate of septic reactions related to platelet transfusions is estimated to be 1 in 100,000.16 Blood components can be contaminated by gram-positive (e.g., Staphylococcus aureus, Staphylococcus epidermidis, Bacillus cereus) or gram-negative (e.g. Escherichia coli, Serratia, Klebsiella pneumonia, Pseudomonas) bacteria. Contamination can result in fever, tachycardia, and hypotension. If bacterial contamination (BACON) is suspected, the transfusion must be stopped immediately, recipient and unit cultures requested, and empiric antibiotics may be administered. It is important to notify the blood bank, who will notify Canadian Blood Services in case there are components from the same donor that need to be removed from inventory.
Multiple strategies are used at Canadian Blood Services to mitigate the risk of bacterial contamination, such as disinfection of donor venesection sites, removal of the first aliquot of collected blood, and culture-based detection systems.17 More information on platelet bacterial testing is available in an FAQ on Canadian Blood Services platelet bacterial screening. Pathogen inactivation adds an extra layer of protection against a number of pathogens, including bacteria and viruses. By effectively damaging the nucleic acids of pathogens, pathogen inactivation further reduces the risk of pathogen transmission. See Chapter 19 for more about pathogen-reduced platelets.
Dyspnea
Dyspnea or hypoxemia can occur during transfusion. Two important transfusion-related causes of this symptom include transfusion-associated circulatory overload (TACO) and transfusion-related acute lung injury (TRALI).
Transfusion-associated circulatory overload
TACO is a significant cause of transfusion-related morbidity and mortality.5 It is characterized by shortness of breath and evidence of volume overload. The reporting criteria for TACO were updated in 2018 by the International Society of Blood Transfusion (ISBT) Working Party on Haemovigilance in collaboration with the International Haemovigilance Network (IHN) and Association for the Advancement of Blood & Biotherapies (AABB) (see Table 3). Many factors are thought to underlie the pathophysiology of TACO, such as increased hydrostatic pressure from volume overload and increased fluid filtration due to inflammation.18 It is important to identify patients at risk for TACO and consider preventative measures such as minimizing transfusion, slower infusions, or pre-transfusion diuretics. If TACO is suspected, the transfusion must be stopped and diuretics given to address the pulmonary edema. Supportive measures such as supplemental oxygen should also be provided.
Table 3. Revised TACO case surveillance definition from the ISBT Working Party on Haemovigilance in collaboration with the IHN and AABB.19
| Revised ISBT-IHN-AABB TACO case surveillance definition (2018) | |
|---|---|
|
Patients classified with a TACO (surveillance diagnosis) should exhibit at least one required criterion* with onset during or up to 12 hours after transfusion and a total of three or more criteria: * Required criteria |
|
| A | Acute or worsening respiratory compromise and/or |
| B |
Evidence of acute or worsening pulmonary edema based on:
|
| C | Development of cardiovascular system changes not explained by the patient’s underlying medical condition, including development of tachycardia, hypertension, jugular venous distension, enlarged cardiac silhouette, and/or peripheral edema |
| D | Evidence of fluid overload including any of the following: a positive fluid balance, clinical improvement following diuresis |
| E | Supportive result of a relevant biomarker, e.g., an increase of B-type natriuretic peptide levels (BNP or NT-pro BNP) above the age group-specific reference range and greater than 1.5 times the pretransfusion value. |
| *A and/or B, and a total of at least three (A to E | |
Transfusion-related acute lung injury
TRALI is a condition that is characterized by non-cardiogenic pulmonary edema and respiratory failure. It is a leading cause of transfusion-related morbidity and mortality.18 The proposed pathophysiological mechanisms of TRALI are not completely clear, but a two-hit mechanism has been hypothesized. A patient’s underlying clinical condition predisposes them to TRALI and this constitutes the first “hit.” The second “hit” is related to the transfused blood component (e.g., donor human leukocyte antibodies directed against recipient cognate antigens or biological response modifiers within the transfused unit).18 For additional information regarding TRALI, including diagnosis and management, please see the Canadian Blood Services publication, Transfusion-related acute lung injury (TRALI).
A revised definition of TRALI following the original set out by the Canadian Consensus Conference Panel in 2004 has been proposed as outlined in Table 4.
Table 4. New proposed consensus redefinition of TRALI20
| TRALI Type I |
Patients do not have risk factors for Acute Respiratory Distress Syndrome (ARDS) and meet the following criteria: a) i. Acute onset ii. Hypoxemia (P/F ≤ 300 or SpO2 < 90% on room air) iii. Clear evidence of bilateral pulmonary edema on imaging iv. No evidence of left arterial hypertension (LAH), or if LAH is present, it is judged to not be the main contributor to the hypoxemia. b) During or within 6 hours of transfusion c) No temporal relationship to an alternative risk factor for ARDS |
| TRALI Type II |
Patients who have risk factors for ARDS (but who have not been diagnosed with ARDS) or who have existing mild ARDS (PaO2/FiO2* ratio of 200–300), but whose respiratory status deteriorates and is judged to be due to transfusion based on: a) Findings as described in categories a and b of TRALI Type I, and b) Stable respiratory status in the 12 hours before transfusion |
| *PaO2: Partial pressure of oxygen; FiO2: Fraction of inspired oxygen | |
Although the initial presentation of TACO and TRALI can be similar, distinguishing between these two entities is important as the management of TACO and TRALI is different (see Table 5).
Table 5. Comparing and contrasting features of TACO and TRALI
| TACO | TRALI | |
|---|---|---|
| Onset | Occurs during or up to 12 hours after the transfusion | Occurs during or within 6 hours of completion of the transfusion |
| Fever | Typically absent, but can be present6 | May be present |
| Pulmonary edema | Yes | Yes |
| Blood pressure | May be increased | May be decreased |
| Evidence of volume overload | Yes | Inconsistent |
| Responds to diuretics | Yes | Inconsistent |
| Elevated BNP (brain natriuretic peptide) | Yes | Inconsistent |
Transfusion associated dyspnea (TAD)
Transfusion associated dyspnea (TAD) has not been well characterized at this time.21 As per the National Healthcare Safety Network (NHSN), TAD is defined as acute respiratory distress that occurs within 24 hours of a transfusion and does not fit the definitions of TACO, TRALI, or an allergic reaction.22
Allergic reactions
The exact pathophysiology of allergic reactions is unclear, but reactions may be related to specific plasma proteins exposures.23, 24 Food allergens and chemical allergens have also been documented in causing allergic reactions.25 Lastly, others have hypothesized that there are allergen-independent pathways and inflammatory mediators in blood components could trigger an allergic reaction.23
Minor allergic reaction
Minor allergic reactions present with urticaria, pruritus, flushing, mild upper respiratory symptoms, or gastrointestinal symptoms.26They are more common following platelet and plasma transfusion. Minor allergic reactions are usually self-limited and transfusions can often be restarted at a slower rate if there is no evidence of a severe allergic reaction. Pre-medication with acetaminophen and diphenhydramine has not clearly shown a reduction in allergic transfusion reactions.27 Other possible preventative measure can include modification of the blood components (e.g., plasma depletion, washing) for patients with repeated reactions. If a minor allergic reaction has occurred, antihistamines can be given for treatment.
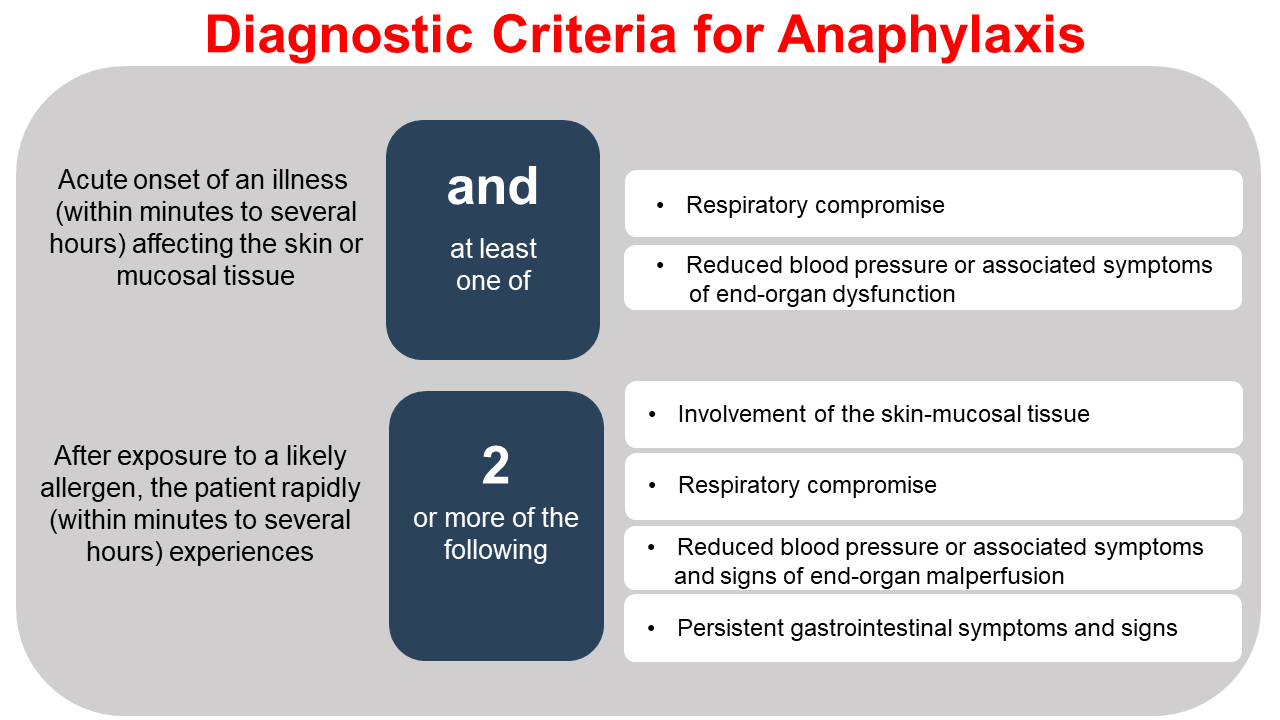
Anaphylaxis
Anaphylaxis is a rare, systemic, and serious complication of transfusion with blood components. The estimated incidence is 1 in 50,000 RBC transfusions.28 Examples of the diagnostic criteria for anaphylaxis are listed below (Figure 2).29
Acute management should focus on maintaining the patient’s airway, breathing, and circulation. Epinephrine, antihistamines, steroids, and inhaled beta-2 agonists can be considered. It is important to keep a broad differential diagnosis in mind based on the patient’s symptoms and take this into account when considering investigations. For example, in the setting of hypotension, one should also consider sepsis, a bradykinin-mediated reaction, or acute hemolysis. If respiratory distress occurs, other causes of dyspnea such as TACO and TRALI should be kept in mind.
IgA deficiency with anti-IgA has previously been thought to be related to allergic reactions.30 However, recent data shows that the association between IgA deficiency with anti-IgA and anaphylactic transfusion reactions is not robust28 (see the Canadian Blood Services publication, Anaphylactic transfusion reactions and IgA deficiency, for more details). In contrast, anaphylactic reactions have been associated with haptoglobin deficiency and those with anti-haptoglobin antibodies.31 Lastly, there have been case reports of anaphylactic reactions in the context of passive IgE transfer32 by transfusion and through the transfer of food allergens in blood products.33
Hypotension
There are multiple potential causes of hypotension in the context of transfusion of blood components. Bacterial contamination, TRALI, anaphylaxis, and acute hemolytic transfusion reactions can manifest with hypotension. Hypotension may also be due to reasons unrelated to the transfusion such as underlying bleeding and exsanguination. To that end, it is important to carry out a thorough assessment of the patient and the accompanying symptoms to further narrow the differential.
Bradykinin-mediated hypotension
Hypotensive events have also been associated with bradykinin, an inflammatory molecule that mediates smooth muscle contraction and vasodilation.34 The Transfusion Transmitted Injuries Surveillance System (TTISS) considers a drop in systolic blood pressure of ≥ 30 mmHg and a systolic blood pressure below 80mmHg to be a hypotensive reaction.35 The interaction between Factor XII in plasma with negatively charged surfaces (blood filters, dialysis membranes, tubing systems) can result in the production of bradykinin.9 Angiotensin-converting enzyme (ACE) can inactivate bradykinins; therefore, ACE inhibitors can result in higher amounts of bradykinin and increase the risk of hypotension.34 The occurrence of hypotension during apheresis in patients who are on ACE-inhibitor therapy has been well-documented,36, 37 and the medication should be stopped prior to the procedure. If bradykinin-mediated hypotension is suspected, the transfusion should be stopped and IV fluids should be provided if necessary.
Delayed cytopenias
After a transfusion of one adult dose of platelets, an increment of approximately 15–25 x 109/L in platelet count can be expected (in the absence of consumption/sequestration).38 With RBC transfusion, one unit typically results in an increment of 10 g/L in hemoglobin concentration (in the absence of ongoing blood loss/hemolysis). Although rare, it is possible that recipients of blood components can experience a paradoxical decrease in their counts. Delayed hemolytic transfusion reactions, transfusion-associated graft versus host disease (TA-GvHD), post-transfusion purpura (PTP), and transfusion-related alloimmune neutropenia can result in cytopenias.
Delayed hemolytic transfusion reaction
Delayed hemolytic transfusion reactions (DHTR) tend to occur more than 24 hours after the transfusion. This reaction can present with jaundice, low-grade fever, and laboratory evidence of hemolysis (elevated LDH, decreased haptoglobin, and a positive direct antiglobulin test). The median time of presentation is 1–2 weeks after the transfusion. They can be related to RBC alloantibodies more commonly, anti E, JKa, c, Fya, and K, though many other alloantibodies can cause hemolysis).39 Investigations of a DHTR can include phenotyping on a pre-transfusion sample, phenotyping of the transfused RBCs for the suspected antigen, and performing an eluate from a post-transfusion patient RBC sample. DHTRs can be prevented through multiple strategies, including transfusing only when necessary, repeating antibody screens, maintaining antibody history in patient records, sharing information between blood banks, and prophylactic antigen matching for certain populations. For example, the International Collaboration for Transfusion Medicine Guidelines (ICTMG) recommends that patients with sickle cell disease receive blood that is matched for Rh and K (C, E, c, e, K) and that extended matching for Kidd, Duffy and S/s antigens should also be considered in those with any alloantibodies.40
Hemolysis can be driven by non-immune causes. For example, heating of RBC to a temperature greater than 45°C has been associated with hemolysis.41 Mechanical hemolysis caused by transfusion through small gauge needles42 or transfusion through clogged filters may also occur. Finally, hemolysis can occur when blood components are outdated or improperly stored.
Post-transfusion purpura
Post-transfusion purpura (PTP) manifests as severe thrombocytopenia within 2 weeks of transfusion.43 Due to hemorrhagic complications, PTP is associated with a mortality rate of 10–20%.44 The underlying mechanisms of PTP is thought to be related to alloantibodies to human platelet antigens (HPA) that result in the destruction of transfused platelets as well as the patient’s own platelets.11 The exact mechanism that causes autologous platelet clearance is unclear, but the following processes have been proposed:43
-
Removal due to specific and non-specific adherence of immune complexes (e.g., anti-HPA-1a & transfused HPA-1a) that bind to the autologous platelets
-
Platelet autoantibodies produced concomitantly with alloantibodies
The risk of PTP can be lowered through judicious use of blood components and leukoreduction of blood components. Leukoreduction may prevent platelet contamination of red blood cell units and decrease the likelihood of antibody formation.43 For patients with known anti-HPA antibodies, red cell washing or HPA-selected platelets can be considered. The first-line therapy for PTP is intravenous immunoglobulin (IVIG)43 and other potential treatments include steroids or plasmapheresis. Prior to the first IVIG treatment, samples for HPA antibody testing should be collected. Platelet transfusion may not result in meaningful increments in the platelet count even if the component is negative for the offending HPA antigen.43
Transfusion-associated graft versus host disease
TA-GvHD is a very rare transfusion reaction in which transfused, viable donor lymphocytes engraft and attack recipient tissues. The estimated mortality rate of TA-GvHD is greater than 90%.45 The clinical presentation of TA-GvHD can vary and may manifest with rashes, transaminitis, fever, gastrointestinal symptoms, and/or pancytopenia. To diagnose TA-GvHD, a biopsy (e.g., skin) can be helpful. Chimerism can also be assessed.
Risk factors include immunodeficiency which may be driven by underlying malignancies, medications, or hematopoietic cell transplant. HLA selected products and transfusion within populations where there is a high degree of HLA similarity between individuals (e.g., in Japan) may also contribute.46 In these cases, the recipient’s immune system may not be able to appropriately remove donor-T cells due to partial HLA matching. Risk factors can be conceptualized as patient-related and component-related (see Table 6). Notably, a systematic review completed in 2015 found that about half of TA-GvHD cases were found to have occurred in patients that would not have been predicted to be at risk.47
Table 6. Risk factors for TA-GvHD
| Patient-related risk factors | Component-related risk factors |
|---|---|
|
|
To reduce the risk of TA-GvHD, blood components can be irradiated prior to transfusion. Irradiation prevents T-lymphocytes from replicating. More information on irradiated blood components can be found in Chapter 15 on irradiated, washed, and CMV seronegative blood components. Recommendations from the National Advisory Committee on Blood and Blood Products (NAC) provide guidance on scenarios in which irradiated blood components should be provided.10 As irradiation significantly impacts on the quality of stored RBC components, irradiation should be undertaken as close to the time of transfusion as possible. When irradiated RBCs are not available, a 2021 study found that hypothermic storage of RBCs for 21 days or more is sufficient to inactivate T-lymphocytes, which may help prevent TA-GvHD.48 If TA-GvHD occurs, treatment may involve immunosuppression and bone marrow transplant can be considered.
Transfusion-related alloimmune neutropenia
Transfusion-related alloimmune neutropenia (TRAIN) is a very rare side effect that is often associated with TRALI.49 It has been postulated that donor antibodies against neutrophils could be part of the underlying mechanism of this transfusion reaction.50 The estimated incidence of TRAIN is thought to be less than 1 in 10,000.50
Infection
The risk of infection through transmission of blood components is uncommon. To mitigate the risk of transfusion-transmissible agents, donors are screened, and tests are performed on all blood donations (see Chapter 6 for more on transfusion-transmissible disease testing at Canadian Blood Services). Donations are tested for various viruses such as HIV, hepatitis B, hepatitis C, HTLV, and West Nile virus (seasonally or if there is a related travel-risk). Cytomegalovirus serologic testing is also done for components that are used for intrauterine transfusion. The parasite causing Chagas disease, Trypanosoma cruzi, is also tested if the donor is deemed to be at risk based on their questionnaire. All donations are tested for Treponema pallidum, the bacteria responsible for syphilis. Non-pathogen-reduced platelet doses also undergo bacterial screening (please see FAQ: Canadian Blood Services platelet bacterial screening). For more about Canadian Blood Services’ infectious risk monitoring, see the annual surveillance report.
The window period is the interval between the time of infectivity and the appearance of detectable disease markers such as specific antibodies or viral nucleic acid sequences. During the window period there is a risk that a specific disease will be present but not detected. Current window period estimates are:
-
10 days for HIV
-
8 days for HCV
-
38 days for HBV
In January 2022, Canadian Blood Services introduced pathogen-reduced platelets (pooled platelets psoralen-treated or PPPT), a component manufactured using pathogen inactivation technology, at its Ottawa production site. For more information on pathogen-reduced platelets, including mechanism of action for the Cerus INTERCEPT pathogen inactivation technology, read Chapter 19: Pathogen-reduced platelets. For an overview of pathogen inactivation systems being applied to blood components, see Chapter 6: Donor selection, donor testing, and pathogen reduction.
Other
Iron overload
Chronic transfusions may be necessary for patients with various conditions such as hematologic malignancies, congenital hemolytic anemias, aplastic anemia, or other conditions resulting in ineffective erythropoiesis.51 A unit of RBCs is estimated to contain 200–250 mg of iron51 and the risk of deposition into tissues rises once approximately 10–20 units of RBCs are transfused.51 Accumulation of iron in the heart, liver, and endocrine organs can lead to significant organ dysfunction. For example, potential endocrine abnormalities include hypogonadotropic hypogonadism, diabetes mellitus, and osteoporosis.52
A diagnosis of iron overload can be made by assessing liver iron concentration through MRI scans.53 Other tests such as ferritin and transferrin saturation are not as accurate as MRI, but they can be useful tools for monitoring treatment response. Treatment of iron overload varies depending on the underlying cause. In cases where ongoing chronic transfusions are needed, chelation provides a way to reduce the iron burden. Chelators can be administered as oral tablets or as parenteral medications.
Continuing professional development credits
Fellows and health-care professionals who participate in the Canadian Royal College's Maintenance of Certification (MOC) program can claim the reading of the Clinical Guide to Transfusion as a continuing professional development (CPD) activity under Section 2: Individual learning. Learners can claim 0.5 credits per hour of reading to a maximum of 30 credits per year.
Medical laboratory technologists who participate in the Canadian Society for Medical Laboratory Science’s Professional Enhancement Program (PEP) can claim the reading of the Clinical Guide to Transfusion as a non-verified activity.
Acknowledgements
The authors acknowledge Michelle Zeller, MD, FRCPC, MHPE, DRCPSC, for her review of this chapter.
If you have questions about the Clinical Guide to Transfusion or suggestions for improvement, please contact us through the Feedback form.
Suggested citation
Laureano M, Khandelwal A, Yan M. Transfusion reactions. In: Khandelwal A, Abe T, editors. Clinical Guide to Transfusion [Internet]. Ottawa: Canadian Blood Services, 2022 [cited YYYY MM DD]. Chapter 10. Available from: https://professionaleducation.blood.ca
References
- Public Health Agency of Canada. Transfusion Transmitted Injuries Surveillance System (TTISS), 2018-2019. Vol. 2022 (Government of Canada, 2018).
- World Health Organization. A guide to establishing a national haemovigilance system. Vol. 2022 (World Health Organization, 2018).
- Appelbaum, P.S. Assessment of Patients' Competence to Consent to Treatment. New England Journal of Medicine 357, 1834-1840 (2007).
- Canadian Medical Protective Association. Consent: A guide for Canadian physicians. (CMPA, 2021).
- Public Health Agency of Canada. Transfusion Transmitted Injuries Surveillance System 2011 - 2015 Summary Report (2015).
- Geiger, T.L. & Howard, S.C. Acetaminophen and Diphenhydramine Premedication for Allergic and Febrile Nonhemolytic Transfusion Reactions: Good Prophylaxis or Bad Practice? Transfusion medicine reviews 21, 1-12 (2007).
- Roubinian, N. TACO and TRALI: biology, risk factors, and prevention strategies. Hematology 2018, 585-594 (2018).
- Bulle, E.B., Klanderman, R.B., Pendergrast, J., et al. The recipe for TACO: A narrative review on the pathophysiology and potential mitigation strategies of transfusion-associated circulatory overload. Blood reviews 52, 100891 (2022).
- Choosing Wisely Canada. Ten tests and treatments to question in transfusion medicine. Vol. 2022 (Choosing Wisely Canada,, 2021).
- National Advisory Committee on Blood and Blood Products. Recommendations for use of irradiated blood components in Canada: A NAC and CCNMT collaborative initiative. (2018).
- Cohen, R., Escorcia, A., Tasmin, F., et al. Feeling the burn: the significant burden of febrile nonhemolytic transfusion reactions. Transfusion 57, 1674-1683 (2017).
- Wang, S.E., Lara, P.N., Jr., Lee-Ow, A., et al. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double-blind placebo-controlled trial. Am J Hematol 70, 191-194 (2002).
- Panch, S.R., Montemayor-Garcia, C. & Klein, H.G. Hemolytic Transfusion Reactions. New England Journal of Medicine 381, 150-162 (2019).
- Marconi, M., Langeberg, A.F., Sirchia, G., et al. Improving transfusion safety by electronic identification of patients, blood samples, and blood units. Immunohematology / American Red Cross 16, 82-85 (2000).
- Ramirez-Arcos, S., Evans, S., McIntyre, T., et al. Extension of platelet shelf life with an improved bacterial testing algorithm. Transfusion 60, 2918-2928 (2020).
- Ramirez-Arcos, S., DiFranco, C., McIntyre, T., et al. Residual risk of bacterial contamination of platelets: six years of experience with sterility testing. Transfusion 57, 2174-2181 (2017).
- Palavecino, E.L., Yomtovian, R.A. & Jacobs, M.R. Bacterial contamination of platelets. Transfusion and apheresis science: Official journal of the World Apheresis Association: Official journal of the European Society for Haemapheresis 42, 71-82 (2010).
- Semple, J.W., Rebetz, J. & Kapur, R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood 133, 1840-1853 (2019).
- Schipperus, M.R., Wiersum-Osselton, J.C. & Group, O.B.o.t.I.-I.-A.T.D.R. Updated definitions for respiratory complications of blood transfusion. Transfusion 59, 2482-2483 (2019).
- Vlaar, A.P.J., Toy, P., Fung, M., et al. A consensus redefinition of transfusion-related acute lung injury. Transfusion 59, 2465-2476 (2019).
- Badami, K.G., Joliffe, E. & Stephens, M. Transfusion-associated dyspnoea – shadow or substance? Vox Sanguinis 109, 197-200 (2015).
- Centers for Disease Control and Prevention. National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol. (Atlanta, GA, 2021).
- Hirayama, F. Current understanding of allergic transfusion reactions: incidence, pathogenesis, laboratory tests, prevention and treatment. British Journal of Haematology 160, 434-444 (2013).
- Khandelwal, A., Clarke, G., Goldman, M. Anaphylactic transfusion reactions and IgA deficiency (Canadian Blood Services, Ottawa, 2021).
- Ching, J.C.Y., Lau, W., Hannach, B., et al. Peanut and fish allergy due to platelet transfusion in a child. CMAJ : Canadian Medical Association Journal 187, 905-907 (2015).
- Adkins, B.D., Lawicki, S., Johnson, M., et al. Mild Allergic Transfusion Reactions: Impact of Associated Clinical Symptoms? American Journal of Clinical Pathology 151, 344-348 (2019).
- Kennedy, L.D., Case, L.D., Hurd, D.D., et al. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion 48, 2285-2291 (2008).
- Sandler, S.G., Eder, A.F., Goldman, M., et al. The entity of immunoglobulin A-related anaphylactic transfusion reactions is not evidence based. Transfusion 55, 199-204 (2015).
- Sampson, H.A., Muñoz-Furlong, A., Campbell, R.L., et al. Second symposium on the definition and management of anaphylaxis: Summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Journal of Allergy and Clinical Immunology 117, 391-397 (2006).
- Schmidt, A.P., Taswell, H.F. & Gleich, G.J. Anaphylactic Transfusion Reactions Associated with Anti-IgA Antibody. New England Journal of Medicine 280, 188-193 (1969).
- Shimada, E., Tadokoro, K., Watanabe, Y., et al. Anaphylactic transfusion reactions in haptoglobin-deficient patients with IgE and IgG haptoglobin antibodies. Transfusion 42, 766-773 (2002).
- Arnold, D.M., Blajchman, M.A., DiTomasso, J., et al. Passive Transfer of Peanut Hypersensitivity by Fresh Frozen Plasma. Archives of Internal Medicine 167, 853-854 (2007).
- Jacobs, J.F.M., Baumert, J.L., Brons, P.P., et al. Anaphylaxis from passive transfer of peanut allergen in a blood product. The New England Journal of Medicine 364, 1981-1982 (2011).
- Cyr, M., Eastlund, T., Blais, C., et al. Bradykinin metabolism and hypotensive transfusion reactions. Transfusion 41, 136-150 (2001).
- Public Health Agency of Canada. Transfusion Transmitted Injuries Surveillance System. User’s Manual. Version 3.0 (2007).
- Owen, H.G. & Brecher, M.E. Atypical reactions associated with use of angiotensin-converting enzyme inhibitors and apheresis. Transfusion 34, 891-894 (1994).
- Fried, M.R., Eastlund, T., Christie, B., et al. Hypotensive reactions to white cell-reduced plasma in a patient undergoing angiotensin-converting enzyme inhibitor therapy. Transfusion 36, 900-903 (1996).
- The Trial to Reduce Alloimmunization to Platelets Study Group. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. The New England Journal of Medicine 337, 1861-1869 (1997).
- Pineda, A.A., Vamvakas, E.C., Gorden, L.D., et al. Trends in the incidence of delayed hemolytic and delayed serologic transfusion reactions. Transfusion 39, 1097-1103 (1999).
- Compernolle, V., Chou, S.T., Tanael, S., et al. Red blood cell specifications for patients with hemoglobinopathies: a systematic review and guideline. Transfusion 58, 1555-1566 (2018).
- Gershfeld, N.L. & Murayama, M. Thermal instability of red blood cell membrane bilayers: temperature dependence of hemolysis. J Membr Biol 101, 67-72 (1988).
- Miller, M.A. & Schlueter, A.J. Transfusions via hand-held syringes and small-gauge needles as risk factors for hyperkalemia. Transfusion 44, 373-381 (2004).
- Hawkins, J., Aster, R.H. & Curtis, B.R. Post-Transfusion Purpura: Current Perspectives. Journal of Blood Medicine 10, 405-415 (2019).
- Shtalrid, M., Shvidel, L., Vorst, E., et al. Post-transfusion purpura: a challenging diagnosis. Isr Med Assoc J 8, 672-674 (2006).
- Vamvakas, E.C. & Blajchman, M.A. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood 113, 3406-3417 (2009).
- Shivdasani, R.A., Haluska, F.G., Dock, N.L., et al. Graft-versus-Host Disease Associated with Transfusion of Blood from Unrelated HLA-Homozygous Donors. New England Journal of Medicine 328, 766-770 (1993).
- Kopolovic, I., Ostro, J., Tsubota, H., et al. A systematic review of transfusion-associated graft-versus-host disease. Blood 126, 406-414 (2015).
- Mykhailova, O., Turner, T.R., Olafson, C., et al. Hypothermic storage of leukoreduced red blood cells for greater than 21 days is a safe alternative to irradiation. Transfusion, 1-11 (2021).
- Hauck-Dlimi, B., Ruppel, R., Zimmermann, R., et al. Transfusion-related alloimmune neutropenia with no pulmonary complications: one donor-five cases. Transfusion 56, 84-90 (2016).
- Wallis, J.P., Haynes, S., Stark, G., et al. Transfusion-related alloimmune neutropenia: an undescribed complication of blood transfusion. Lancet 360, 1073-1074 (2002).
- Remacha, Á., Sanz, C., Contreras, E., et al. Guidelines on haemovigilance of post-transfusional iron overload. Blood Transfusion 11, 128-139 (2013).
- Kim, M.K., Lee, J.W., Baek, K.H., et al. Endocrinopathies in transfusion-associated iron overload. Clin Endocrinol (Oxf) 78, 271-277 (2013).
- Coates, T.D. Iron overload in transfusion-dependent patients. Hematology 2019, 337-344 (2019).