A Guide to Reporting Adverse Transfusion Reactions
Author: Matthew Yan, MD, FRCPC
Publication date: January 2020
Primary target audience: health-care professionals working in hospitals in Canada, excluding Quebec
The following is a guide for reporting adverse transfusion reactions. This guide applies to hospitals in Canada, excluding Quebec. To learn more about the diagnosis, classification and management of transfusion reactions, please refer to Chapter 10 of Canadian Blood Services’ Clinical Guide to Transfusion and the TTISS User Manual (version 3.0 2007). This guide does not cover error and accident reporting. For more information on error and accident reporting, please refer to sections 103–108 of the Blood Regulations.
Provincial Reporting Processes
Please always refer to your provincial policies on transfusion reaction reporting. Although similar practices occur nationally, there may be small differences by province (e.g. reporting of minor reactions such as febrile non-hemolytic transfusion reactions and use of different transfusion reaction forms).
Links to provincial blood coordinating offices and their respective sections on reporting adverse transfusion reactions can be found here:
- BC Provincial Blood Coordinating Office
- Alberta Provincial Blood Coordinating Program
- Saskatchewan Provincial Blood Coordinating Office
- Best Blood Manitoba
- Ontario Regional Blood Coordinating Network
- Newfoundland and Labrador Provincial Blood Coordinating Program
- Nova Scotia Provincial Blood Coordinating Program
Canadian National Hemovigilance System
In response to the 1997 Report of the Commission of Inquiry on the Blood System in Canada by Justice Krever, several initiatives were launched to improve the surveillance of the Canadian blood system. One of these initiatives is the Transfusion Transmitted Injuries Surveillance System (TTISS). Launched in 2001 by the Centre for Communicable Disease and Infection Control (CCDIC) of the Public Health Agency of Canada (PHAC), TTISS is a pan-Canadian surveillance system that captures adverse transfusion reactions to blood components (i.e. red blood cells, platelets, plasma) and plasma protein and related products. Although participation is voluntary at the national level (some provinces have mandated reporting), approximately 80–100 percent of hospitals within all provinces and territories report into TTISS.
Blood components
In addition to voluntary reporting to TTISS, under the Blood Regulations an unexpected adverse reaction or serious adverse reaction must be reported to Health Canada (directly by the hospital or via the manufacturer) if there is concern of risk to human safety or the safety of the blood component. Please refer to the algorithm below (Figure 1) for more information on which reactions may potentially suggest a risk to human safety or safety of the blood component. Hospitals must report directly to Health Canada’s Canada Vigilance Program if regulated activities at the local blood bank may have contributed to the reaction, or they must report to the manufacturer (Canadian Blood Services, Héma-Québec) if regulated activities at the manufacturer may have contributed to the reaction. This allows the manufacturer to complete necessary investigations and further report to Health Canada’s Canada Vigilance Program.
Plasma protein and related products
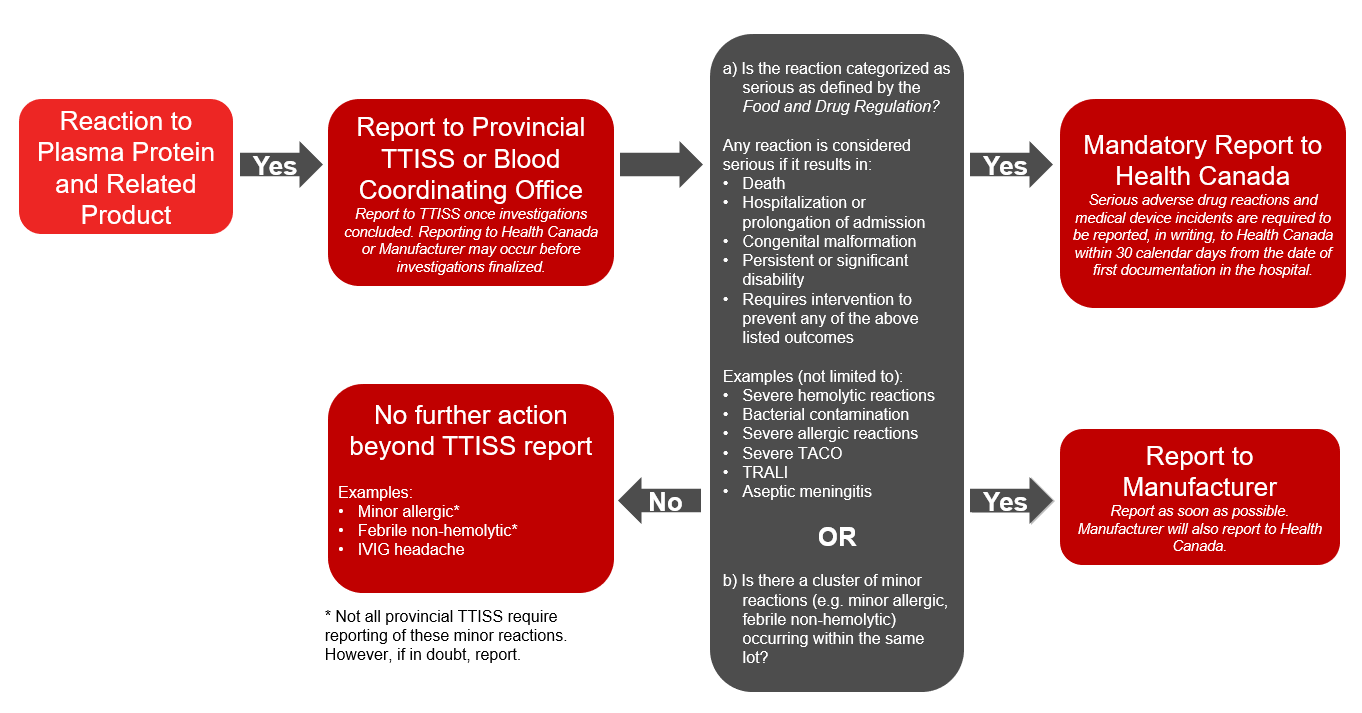
Plasma protein and related products refers to any fractionated protein products, recombinant products or pharmaceuticals (e.g., emicizumab) on the Canadian Blood Services formulary. In addition to voluntary reporting to TTISS, under the Food and Drug Regulations hospitals must report a serious adverse drug reaction associated with transfused plasma protein and related products to Health Canada’s Canada Vigilance Program. A report should also be submitted to the manufacturer. The Protecting Canadians from Unsafe Drugs Act (Vanessa’s Law), an amendment to the Food and Drugs Act which became effective on December 16, 2019, mandates the reporting of all serious adverse reactions to plasma protein and related products to Health Canada. Please refer to the algorithm below (Figure 2) for more information on reporting reactions to plasma protein and related products.
Understandably, the landscape of reporting can be confusing given all the pathways of reporting and interested stakeholders. Below is a guide to assist in the navigation of transfusion reaction reporting. As a general rule, if there is ever any doubt on whether reporting is required, it is better to over-report than under-report.
Why report?
For many reasons, it is important that transfusion services report adverse transfusion reactions:
- It may result in product recall of co-components from the same donor.
- It may result in donor notification and/or investigation and/or deferral.
- It may result in recipient notification and investigation.
- It is useful for purposes of tracking and trending (for example, a new complication or an unexpected change in frequency of a previously recognized complication).
- It contributes to safer transfusion medicine practice.
To whom and how do we report?
- Health Canada’s Canada Vigilance Program: Please refer to the Canada Vigilance Program website.
- Public Health Agency of Canada’s (PHAC) TTISS: Please report to your provincial TTISS office or provincial blood office, which will forward the reaction report to PHAC. Hospitals in smaller provinces, such as P.E.I., may report directly to PHAC.
- Canadian Blood Services: Please contact your local Canadian Blood Services distribution centre.
- Manufacturers of plasma protein and related products: Please contact the relevant manufacturer.
For reporting forms, please see below.
General algorithm for reporting
Please always refer to your provincial policies on transfusion reaction reporting. The algorithms below only serve to act as a guide.
In general, most or all reactions should be reported to your provincial TTISS or blood coordinating office. In addition, further reporting is required in these cases:
- If the reaction is to a component, is serious or unexpected, and is due to a regulated activity, further report to Health Canada or Canadian Blood Services depending on where the activity occurred.
- If the reaction is to a plasma protein and related product and is classified as serious or if there is a cluster of minor reactions associated with the same lot, further report to Health Canada and the manufacturer.
Figure 1: Reporting adverse reactions: algorithm for blood components
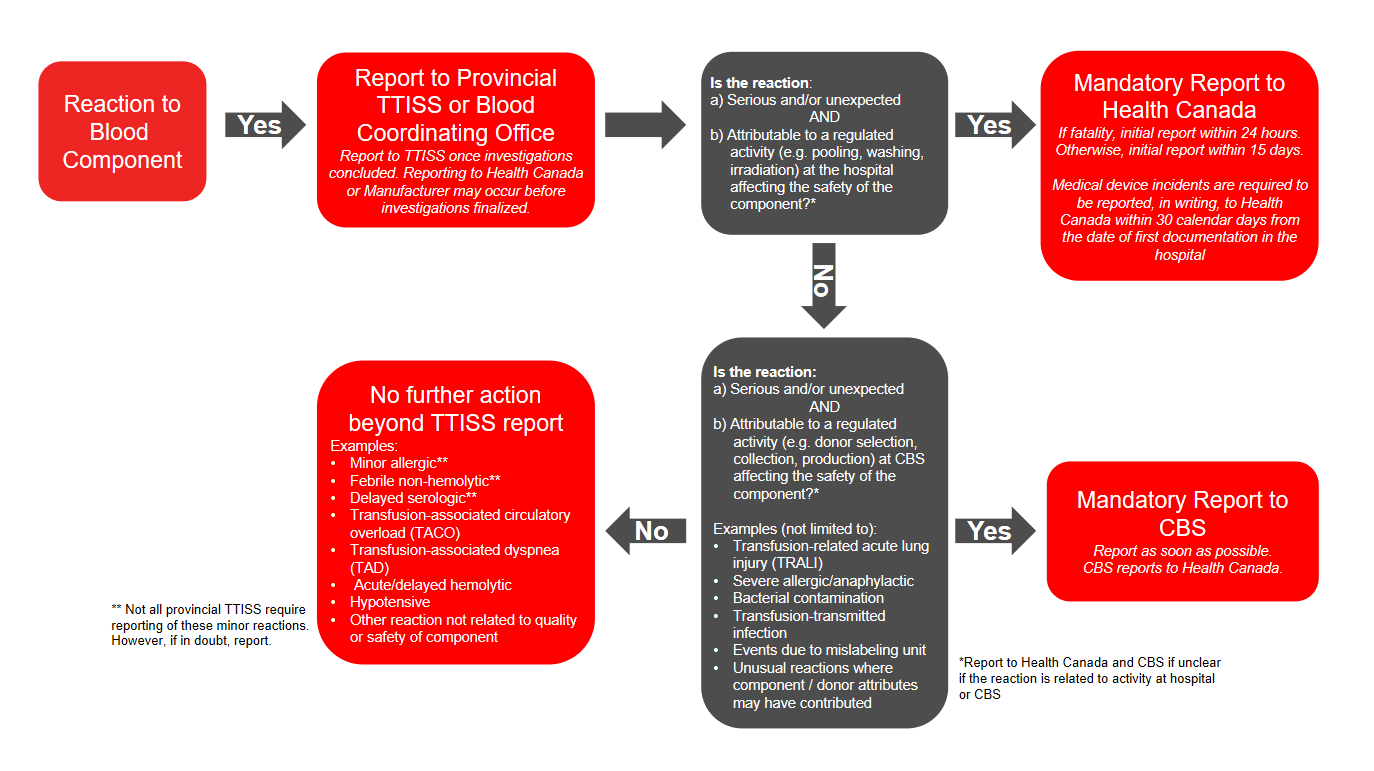
Figure 2: Reporting adverse reactions: algorithm for plasma protein and related products
Reporting forms
Hospital and provincial forms for reporting into TTISS may vary. Please refer to your provincial TTISS or blood coordinating office to determine which form is accepted. The Canadian Transfusion Adverse Event Reporting Form (CTAERF) is available for use and can be found here. For ease of reporting, the manufacturers (including Canadian Blood Services) and Health Canada’s Canada Vigilance Program will accept the CTAERF or any provincial form.
Mandatory reporting of a serious adverse reaction to a transfused plasma protein and related product can also be submitted to Health Canada’s Canada Vigilance Program using the Serious Adverse Drug Reaction Reporting Form for Hospitals. This is also available as an online fillable form with electronic submission (the online form also provides guidance for submitting events related to medical devices).
To report a TRALI to Canadian Blood Services, please also include the Canadian Blood Services TRALI Patient Data Form in addition to any provincial forms used for adverse reaction reporting. The TRALI Patient Data Form can be found here.
To report a transfusion-transmitted infection aside from suspected bacterial contamination (e.g. HIV, viral hepatitis), please refer to the Transmissible Disease Notification form or contact your local Canadian Blood Services distribution centre.
Links to reporting forms:
- Serious Adverse Drug Reaction Reporting Form for Hospitals
- CTAERF
- Canadian Blood Services TRALI Patient Data Form
- Transmissible Disease Notification Form
Resources
- Clinical Guide to Transfusion – Chapter 10: Transfusion reactions
- Food and Drugs Act
- Blood Regulations
- Blood Regulations – Guidance Document – Sections 110-116: Adverse Reactions
- Blood Regulations
- Food and Drug Regulations, Part C (Drugs), Division 1
- Mandatory reporting of serious adverse drug reactions and medical device incidents by hospitals – Guidance document
- Public Health Agency of Canada – Transfusion Transmitted Injuries Surveillance System