Chapter 8
Pre-transfusion testing
Background
Pre-transfusion testing ensures compatibility between the transfusion patient and the blood component(s) (red blood cells, platelets, plasma) intended for transfusion. This process includes accurate and thorough completion of the requisition, accurate patient identification, proper collection and labelling of the blood sample from the patient, laboratory testing to determine the patient’s blood group and to identify the presence of red blood cell alloantibodies, and compatibility testing. Pre-transfusion testing is complete when a compatible blood component is identified and labelled for transfusion to the intended patient. This chapter provides an overview of the pre-transfusion tests that are routinely performed.
Request for testing
The first step in pre-transfusion testing is the preparation of the test requisition. Patient information, including full first and last name, unique identification number and requested tests, must be documented legibly. Repeat testing should not be requested if the patient has current valid testing, as described in the following sections. Date, time and dose of intended transfusion should be provided if available, along with any special requirements and clinical indication. 1-3
Patient identification and sample collection and labelling
It is essential that hospitals and outpatient areas collecting samples for pre-transfusion testing have a specific policy and procedure for unequivocal patient identification and appropriate labelling of pre-transfusion blood samples. Patient identification must be verified at time of sample collection.1, 3, 4 Patients should be asked to state their first and last name and date of birth since they may be wearing the wrong identification bracelet.4 If inaccuracies or discrepancies are discovered during the identification process, blood samples shall not be collected until the discordances have been satisfactorily resolved.
The blood samples used for patient testing may be anticoagulated in EDTA (preferred) or clotted from a non-anticoagulated sample, depending on local policy. A non-hemolyzed sample of blood is required for testing. Samples must be labelled in the presence of the patient at the time of collection with the patient’s full name and a unique identification number.1-3 Unique identification numbers most commonly include a hospital identification number or a provincial health insurance number. Standards also require documentation of the date and time of sample collection as well as the identity of the phlebotomist. 1, 3, 4
For patients who have been recently transfused with red blood cells, who have been pregnant within the previous three months, or whose history is uncertain, the pre-transfusion blood sample must be collected within 96 hours prior to transfusion.1-3 This pre-transfusion blood sample may be used to crossmatch blood components up to 96 hours after collection. After 96 hours, a new blood sample must be collected from the patient to ensure results of compatibility tests performed after this time frame are valid, given the possibility of new antibody development in patients exposed to red blood cell antigens by transfusion or pregnancy.5
Test results for pre-admission patients who have their blood samples drawn for compatibility testing several days or weeks before the planned surgical date may be considered valid beyond 96 hours, depending on local policy. This only applies if the patient has not been transfused and/or pregnant in the preceding three months. Under these circumstances, extending the validity of prior test results helps ensure compatible blood can be available on the anticipated surgical date.
Pre-transfusion testing
Pre-transfusion tests include ABO and RhD typing of the patient's red blood cells and an antibody screen with the patient’s plasma. The latter is a method to detect clinically significant non-ABO antibodies to red cell antigens. Compatibility testing, often referred to as the crossmatch, is also performed before a non-emergent transfusion. The crossmatch may either be performed serologically or electronically.
ABO typing
ABO typing involves testing the patient’s red blood cells for the presence of A and B antigens using anti-A and anti-B antisera (forward grouping) as well as testing of the patient’s plasma for the presence of anti-A and anti-B using Type A and Type B red blood cells (reverse grouping). Results of the forward grouping must agree with the reverse grouping before the patient’s ABO type can be considered valid. Reverse grouping is not required on infants under four months of age as any antibodies detected during this time would be maternal in origin. For more information on neonatal pre-transfusion testing please see Chapter 13.
|
Forward Grouping Patient red cells test with |
Reverse Grouping Patient plasma tested with |
|||
|---|---|---|---|---|
| Anti-A | Anti-B | A cells | B cells | Interpretation |
| - | - | + | + | O |
| + | - | - | + | A |
| - | + | + | - | B |
| + | + | - | - | AB |
Rh typing
RhD typing involves testing the patient’s red blood cells for the presence of the RhD antigen with anti-D sera. RhD-negative patients may develop antibodies to the RhD antigen if exposed to RhD-positive red blood cells. It is, therefore, preferable to provide Rh-negative blood components to any RhD-negative individual. In particular, RhD-negative female children and women of child-bearing potential should not be given RhD-positive blood components in routine transfusions because development of antibodies to the D antigen can contribute to hemolytic disease of the fetus and newborn (HDFN) in future pregnancies. For more information about HDFN, please see Chapter 12.
ABO and Rh typing (using a manual technique) typically takes approximately 15 minutes.
Antibody screening
Antibodies to non-ABO antigens may develop in anyone who has been exposed to red blood cell antigens different from their own through pregnancy or transfusion. To detect these antibodies, a sample of the patient's plasma is tested against selected commercial Type O red blood cells that express the majority of clinically significant antigens. For infants under four months of age, the screen may be performed on either infant plasma or maternal plasma.
Many techniques are currently available for performing antibody screening (Figure 1). Some methods involve addition of enhancement reagents such as albumin, low ionic strength saline (LlSS), or polyethylene glycol (PEG). These enhancements may reduce the required incubation time thus shortening the time required for completion of testing and may also enhance the sensitivity of the antibody screen. Some laboratories use gel column agglutination technique with anti-IgG antibody in the column (gel card) or microtiter well plates with bound red blood cell antigens (solid phase) to perform antibody screens. Depending on the technique, screening usually takes 30 to 60 minutes to complete due to the required sample incubation time.
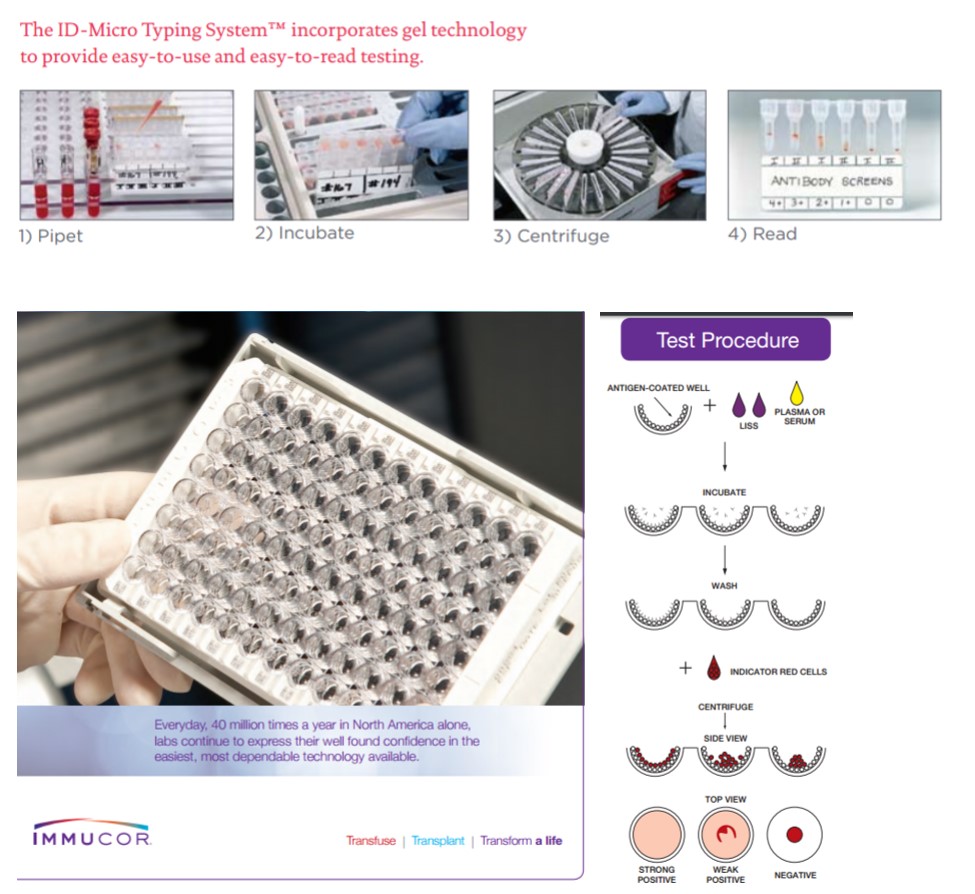
Figure 1. Examples of commercial kits for antibody screening. Top panels: the ID-Micro Typing system from Ortho Clinical Diagnostics. Bottom panels: Immucor Capture-R.
Antibody identification
If antibody screening detects one or more antibodies in a patient's plasma, further testing is performed to identify the antibody(ies). This process, known as antibody identification, involves testing the patient’s plasma with a panel of Type O red blood cells with known antigen expression. Antibody identification involves multiple steps designed to exclude and confirm particular antibodies, determine the optimal temperature of antibody reactivity and identify any autologous reactivity. Extended antigen typing of a patient‘s red blood cells may also assist in antibody identification by establishing which red blood cell antibodies an individual is likely to develop. The time to complete an antibody identification is highly variable and is dependent on the complexity of the antibody investigation required. In some cases antibody identification requires techniques or antisera that may only be available at reference laboratories. In these cases the investigation may take several days.
Crossmatch
Crossmatching is a method of confirming compatibility between the patient and the donor and is the final step in procuring red cell units suitable for transfusion. The crossmatch may either be performed serologically or electronically. In a serologic crossmatch, donor red blood cells are mixed with patient plasma in a test tube or similar testing platform to ensure compatibility. In an electronic (computer) crossmatch, no physical contact between the donor and patient samples is required as a validated computer program ensures that an appropriate blood component has been selected for the intended patient. Electronic crossmatches are only performed when the patient’s current antibody screen is negative and there is no history of a clinically significant antibody.
For a patient who has a clinically significant antibody (or antibodies), the units selected for crossmatch will be antigen-negative. Donor red blood cell units are screened to identify those that lack the antigen(s) corresponding to the antibody(ies) identified in the patient. This screening may involve review of donor phenotyping information printed on the red blood cell unit label in some cases; in other cases, donor units may be phenotyped by the hospital blood bank or requested from Canadian Blood Services (see Chapter 6 of this Guide for more information on red blood cell phenotyping).
If crossmatch-compatible red blood cells cannot be found the transfusion service physician may authorize the release of incompatible units when the need for transfusion outweighs the risk of transfusing serologically incompatible blood. Selection of the most appropriate units for transfusion may be aided by matching the patient red cell antigen phenotype with that of the donor red cells. Depending on the number and complexity of the antibodies present in patient plasma, a variable amount of additional time may be required to find compatible red blood cells. In most cases, compatible allogeneic red blood cells safe for transfusion can be identified.
When compatible blood is very difficult to obtain, rare units or frozen units may be accessed from the blood supplier through the Rare Blood Program.
Type and Screen, or Crossmatch?
A type and screen is ordered if blood transfusion is likely but not certain, while a crossmatch order indicates to the transfusion service that blood transfusion is required.
Ordering a “type and screen” is a common approach to ensuring blood will be available for patients who may require transfusion in a given medical or surgical setting. In the laboratory, a type and screen order results in blood grouping (ABO and Rh typing) and antibody screening. If the screen is negative, a computer crossmatch can confirm compatibility if blood is required. Since this process takes only minutes there is no need to prepare units in advance or tag units of blood for a specific patient. In this setting, if blood is required urgently an ABO and Rh compatible unit is selected by computer crossmatch, followed by labelling and issuing of the blood for transfusion.
If the antibody screen is positive following the type and screen order, then identification of the antibody, selection of antigen-negative donor units and a serological crossmatch are required to ensure blood will be available for transfusion.
For those patients for whom a blood transfusion is intended, a crossmatch should be requested and should include an indication of how many red cell units are required. For a patient with a crossmatch order, in addition to performing a type and screen, compatibility testing is performed along with preparation and labelling of red blood cell units for transfusion.
Compatibility testing may involve a computer (electronic) crossmatch if the antibody screen is negative or a serological crossmatch when an antibody has been identified.
Emergency blood release
When the urgency of the transfusion requirement prevents the initiation or completion of pre-transfusion testing, emergency release of unmatched blood components may be considered. If ABO and RhD testing have been completed, group-specific unmatched red blood cell units may be provided. If no testing has been initiated when blood is required, O-positive or O-negative unmatched red blood cell units will be provided. Type O-negative red blood cells should be conserved in emergencies for female children and women of childbearing potential to prevent overuse of the limited supply of O-negative red blood cell units. A sample for compatibility testing should be obtained as soon as clinical circumstances permit. This allows appropriate switching to group-specific crossmatched blood as soon as possible.
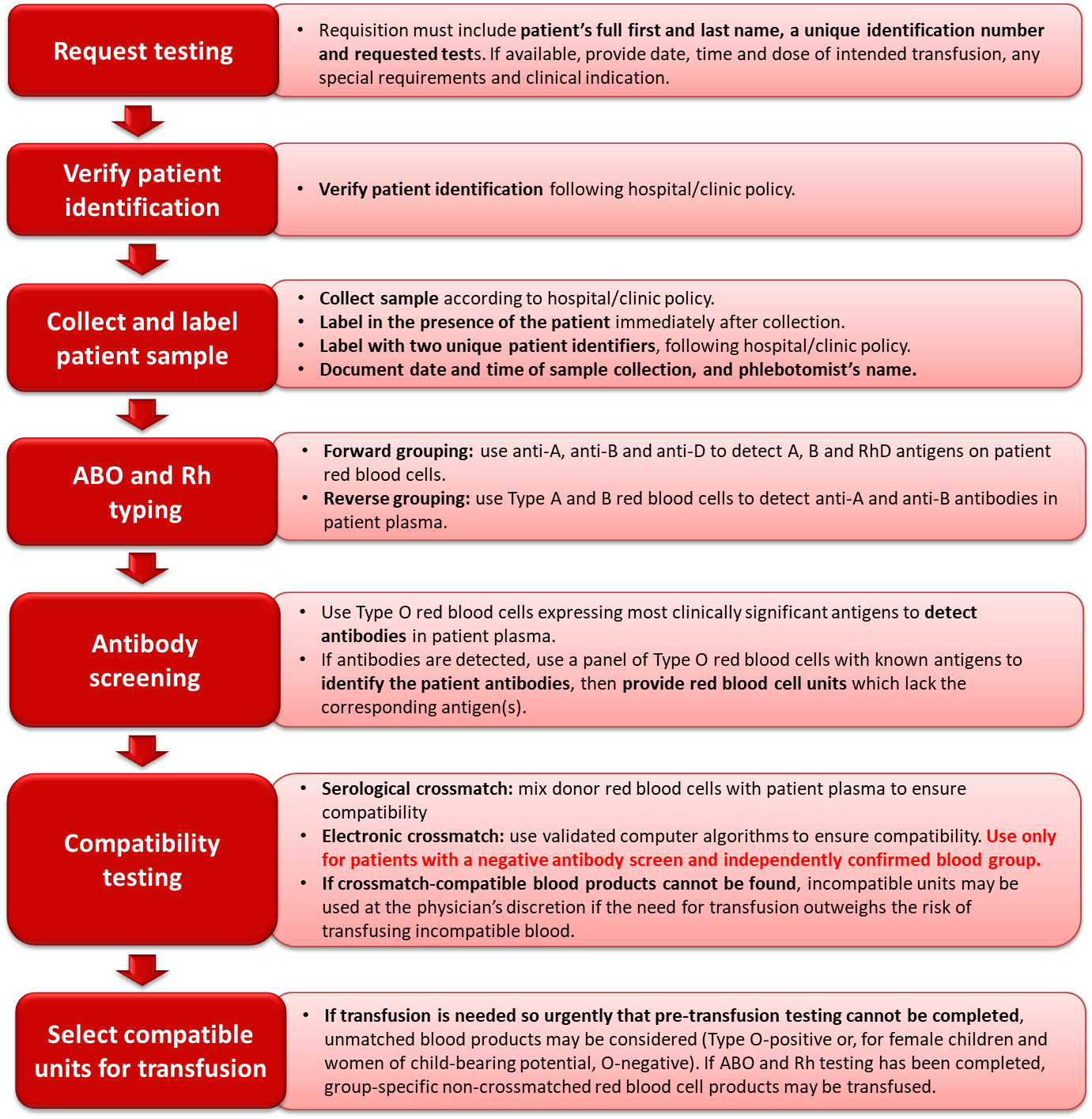
Figure 2. Summary of the pre-transfusion testing process.
Additional resources
For an introduction to immunohematology and the foundations of blood bank compatibility testing, visit LearnSerology.ca, an online educational resource developed by transfusion medicine specialists in Canada. The curriculum consists of six modules and includes an interactive module for completing an antibody investigation panel.
Continuing professional development credits
Fellows and health-care professionals who participate in the Canadian Royal College's Maintenance of Certification (MOC) program can claim the reading of the Clinical Guide to Transfusion as a continuing professional development (CPD) activity under Section 2: Individual learning. Learners can claim 0.5 credits per hour of reading to a maximum of 30 credits per year.
Medical laboratory technologists who participate in the Canadian Society for Medical Laboratory Science’s Professional Enhancement Program (PEP) can claim the reading of the Clinical Guide to Transfusion as a non-verified activity.
Acknowledgements
The author acknowledges Darlene Mueller, MA, ART, and Matthew Yan, MD, FRCPC, for their review of this chapter.
Suggested citation
Duncan J. Pre-transfusion testing. In: Clarke G, Chargé S, editors. Clinical Guide to Transfusion [Internet]. Ottawa: Canadian Blood Services, 2019 [cited 2020 07 10]. Chapter 8. Available from: https://professionaleducation.blood.ca
We’re here to answer your questions about the Clinical Guide to Transfusion. We’d also appreciate your ideas on how to improve the Guide. Please contact us through the Feedback form.
References
- CSTM Standards Committee. CSTM Standards for Hospital Transfusion Services. Published in Ottawa, Canada by Canadian Society for Transfusion Medicine, 2017.
- CSA Group. CAN/CSA-Z902-15 Blood and Blood Components. Published in Canada by CSA, 2015. http://shop.csa.ca/en/canada/blood-and-blood-components/cancsa-z902-15/invt/27020812015.
- The Standards Program Committee and the Blood Banks and Transfusion Services Standards Program Unit. Standards for Blood Banks and Transfusion Services. Published by AABB, 2016
- Murphy MF, Casbard AC, Ballard S, Shulman IA, Heddle N, Aubuchon JP, Wendel S, Thomson A, Hervig T, Downes K, Carey PM, Dzik WH, Collaborative BR. Prevention of Bedside Errors in Transfusion Medicine (Probe-Tm) Study: A Cluster-Randomized, Matched-Paired Clinical Areas Trial of a Simple Intervention to Reduce Errors in the Pretransfusion Bedside Check. Transfusion 2007; 47: 771-80. https://www.ncbi.nlm.nih.gov/pubmed/17465940.
- Shulman IA, Nelson JM, Nakayama R. When Should Antibody Screening Tests Be Done for Recently Transfused Patients? Transfusion 1990; 30: 39-41. https://www.ncbi.nlm.nih.gov/pubmed/2296788.