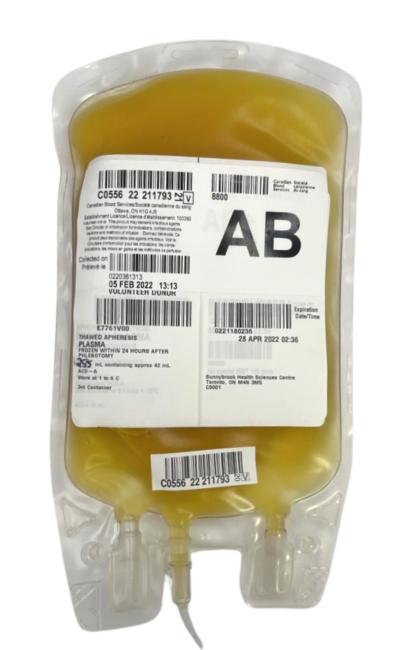
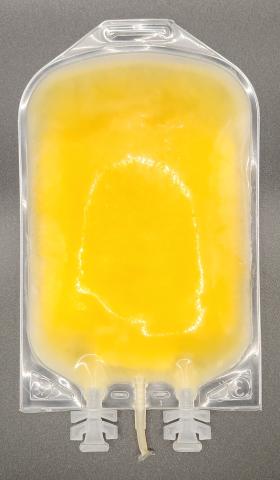
Plasma: Bacterial contamination
Bacterial contamination is the presence of bacteria in the blood. Blood should be free of bacteria. Blood is collected and manufactured under sterile conditions to maintain this aseptic state. Bacterially contaminated blood components should NOT be transfused and any components suspected of bacterial contamination based on a visual inspection should be reported to Canadian Blood Services using the feedback form on blood.ca. Contact the nearest distribution centre to report potential contamination and receive further instructions.
Possible (though rare) causes of bacterial contamination include the following:
- Donor with asymptomatic bacteremia (i.e., subclinical bacteria in blood)
- Inadequate cleansing of skin prior to venipuncture. (Please note: To minimize bacterial contamination, Canadian Blood Services uses a diversion pouch for the first portion of the blood collection. In addition to strict protocol on skin cleansing, the diversion pouch prevents the skin plug and skin bacteria from entering the main unit.)
- Loss of sterility during component manufacturing or handling
Of note, pathogen reduction technologies such as psoralen treatment have been introduced into some blood component manufacturing processes to decrease risk of bacterial contamination/infectious risk.
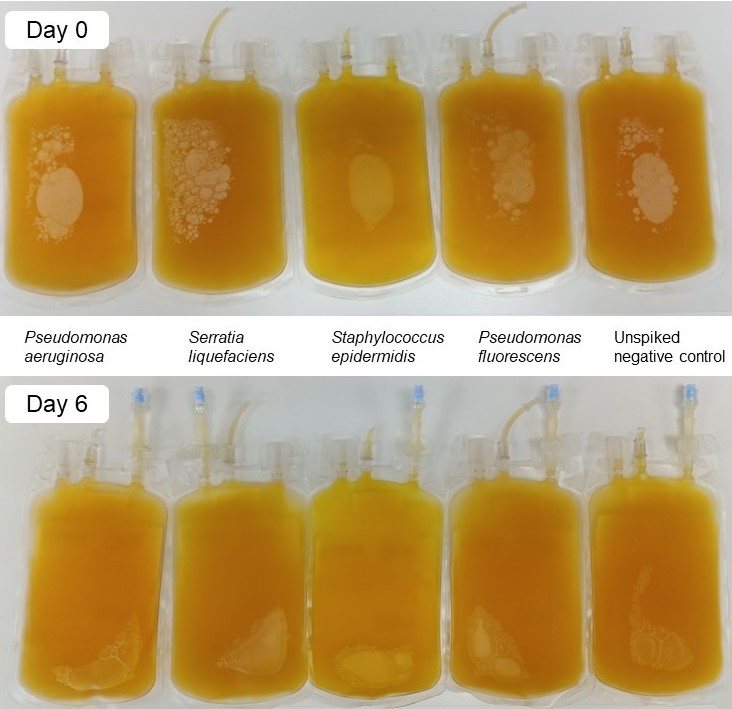
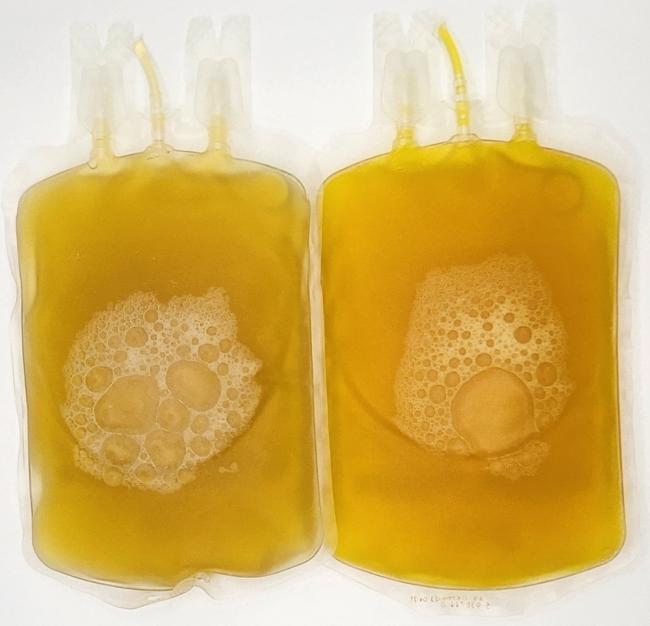
Visual appearance
- The presence of bacteria may produce gas resulting in unusual air bubbles.
- The presence of bacteria may activate clotting resulting in clots and fibrin strands.
- Cloudiness