Chapter 2
Blood components
Background
Each whole blood donation consists of cellular (e.g., red blood cells and platelets) and acellular (e.g., plasma) components. Whole blood is separated into these blood components during the manufacturing process, which allows Canadian Blood Services to offer products to meet patients’ and health-care systems’ diverse needs.
This chapter describes the manufacturing process, indications, contraindications, storage and transportation requirements, dose, administration and available alternatives for the following blood components:
- Red blood cells
- Whole blood, leukocytes reduced (specialized ordering criteria)
- Platelets (a summary of characteristics for all three platelet types currently produced by Canadian Blood Services can be seen in Chapter 19, Table 2, of this Guide):
- Pooled platelets psoralen treated (PPPT)
- Apheresis platelets psoralen-treated (APPT)
- Untreated apheresis platelets in platelet-additive solution (PAS-E)
- Frozen plasma (FP)
- Cryoprecipitate
Further information may be found in other chapters of this Guide as indicated within the different sections.
For additional information on each blood component, review the corresponding Circular of Information which provides comprehensive information about the blood components processed by Canadian Blood Services including component composition, packaging, storage and handling, indications, warnings and precautions, adverse events, dose, and administration. The Circular conforms to the applicable regulations issued by Health Canada.
For information on informed consent for transfusion of blood products and components, see the course entitled Informed consent for blood transfusion on Canadian Blood Services’ professional education website, Profedu.ca.
Collection of blood products
At Canadian Blood Services’ donor centres and mobile donation events, whole blood is collected from donors into a collection pack. The collection pack consists of multiple interconnected bags, which allows components to be transferred aseptically within a closed system during production. The buffy coat collection pack set is used in the production of red blood cell, plasma and platelet components. These collection packs contain citrate-phosphate-dextrose (CPD) which is used as an anticoagulant. Figure 1 highlights the main steps of the buffy coat manufacturing processes.
Canadian Blood Services also uses apheresis technology for the collection of plasma and platelets. This collection procedure utilizes an automated in-line process in which whole blood from the donor enters a collection chamber where centrifugation separates blood constituents (i.e., red blood cells, white blood cells, plasma). Information about the anticoagulants used can be found in the Circular of Information for each component type. Depending on the process, either plasma or platelets suspended in platelet additive solution E (PAS-E) are collected into collection bag(s) while the remaining blood constituents are returned to the donor.
Red blood cells
Component production and description
Whole blood collected in CPD anticoagulant is processed by the Buffy coat method (Figure 1). For this, whole blood is centrifuged to separate the red blood cells from the platelets in buffy coat and plasma. The red blood cells are then leukoreduced by filtration and suspended in an additive solution to enhance their shelf-life and as a source of nutrients.
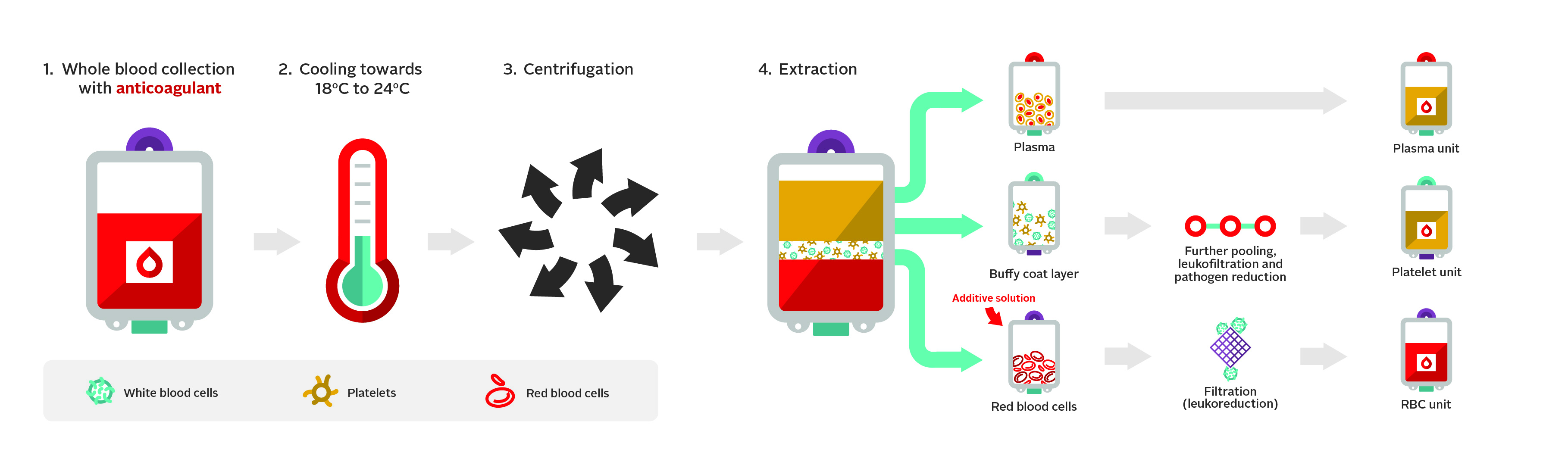
A typical red blood cell unit issued by Canadian Blood Services is 287 mL, contains 55 g of hemoglobin with a hematocrit of approximately 67%, and has an average residual leukocyte count of 6 x 108. Specific parameters are described and updated in the Circular of Information. Further modifications of red blood cell components such as washing and irradiation are covered in Chapter 15 of this Guide.
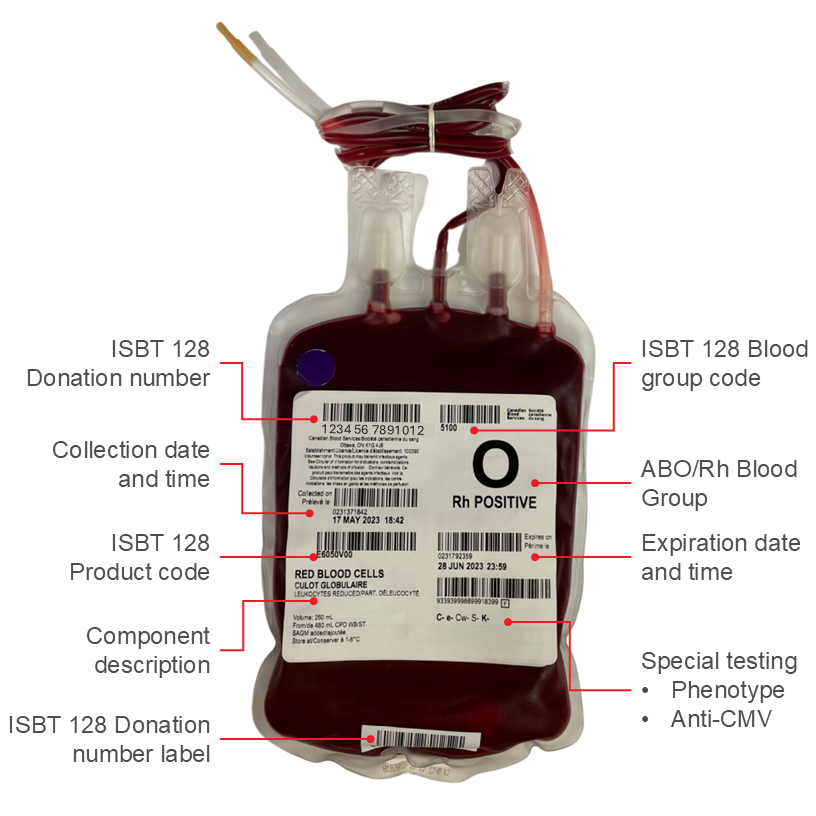
Blood samples collected from the donor at the time of donation are tested for certain transfusion-transmissible infectious agents, see Chapter 6 of this Guide for more information on donor testing and pathogen reduction. All samples for whole blood donations undergo ABO and RhD typing, which is displayed on the component label (Figure 2). Kell typing is also performed on every donor sample and the result is printed on the component label. A proportion of donor samples are phenotyped and/or genotyped for additional red cell antigens corresponding to common clinically significant antibodies (e.g., C, c, E, e, Jka, Jkb, Fya, Fyb, S, s). Negative results for any of these antigens appear on the label while the complete set of results, including positive and negative antigen tests, appears in the bar code label.
Indications
The primary purpose for a red blood cell transfusion is to increase the oxygen-carrying capacity of the blood. Therefore, a red blood cell transfusion is indicated in patients with symptomatic anemia. For example, acute blood loss, chronic symptomatic anemia and cardiopulmonary compromise, or disease or medication effects associated with bone marrow suppression may be triggers for red blood cell transfusion. For management of massive hemorrhage and transfusion support, please see Chapter 11 of this Guide.
Effective oxygen delivery depends not only on the hemoglobin level, but also on the cardiovascular condition of the individual, and the associated ability to compensate for decreased hemoglobin concentration. Patients without cardiopulmonary compromise, therefore, will typically tolerate lower hemoglobin levels than patients with limited cardiopulmonary reserve. Similarly, the normal hemoglobin levels of infants and children vary from those seen in adults and transfusion triggers, as well as blood component dose, vary according to age. Finally, patients who develop anemia slowly develop compensatory mechanisms to allow them to tolerate lower hemoglobin values than patients who become acutely anemic.
The decision to transfuse anemic patients should be based on an individual case assessment. There is no uniformly accepted hemoglobin value below which transfusion should occur for every patient, in every scenario. Although many studies and guidelines support the use of a restrictive transfusion strategy, this is an area of active research, and it is best to refer to the latest red cell guidelines.1 Generally, transfusion may be considered when the hemoglobin falls below 70 g/L among hospitalized patients (including ICU and oncology patients), below 75 g/L in cardiac surgery patients, and below 80 g/L in patients with cardiac ischemia or those undergoing orthopedic surgery.1, 2
Contraindications
Red blood cells are not given for volume replacement and should be given only after non-transfusion alternatives have been either assessed and excluded or do not adequately manage anemia. The decision to transfuse should not be based on a single hemoglobin or hematocrit value as a trigger without considering the clinical status of that patient. Cardiovascular status along with other co-morbid conditions, the acuity and severity of the anemia, and the presence or risk for ongoing blood loss are also considered in determining the need for correction of anemia with transfusion. See the Choosing Wisely Canada website for more information on transfusion guidelines.3
Dose and administration
One unit of red blood cells usually increases hemoglobin by approximately 10 g/L in a 70 kg non-bleeding adult. For pediatric or neonatal patients who require a smaller dose, small volume or “split” units may be provided by some hospital transfusion services.
Chapter 8 and Chapter 9 of this Guide provide detailed information on pre-transfusion testing and administration, respectively. Chapter 13 of this Guide is also recommended for information on neonatal and pediatric transfusion.
If the transfusion of a red blood cell unit will not be initiated promptly after the unit is removed from the temperature-controlled storage, it should be returned to inventory immediately to prevent waste. An unused red blood cell unit may be returned only if the bag is intact, passes a visual inspection and has either been maintained at an acceptable temperature (see Storage and transportation), or has not been out of a temperature-controlled environment for more than 60 minutes (See CSTM Standard 5.8.7.2 for more information).4 Importantly, if temperature controlled coolers are being used by a facility to store or transport the red blood cells, they should be validated to ensure that the red blood cells can remain outside the transfusion laboratory for the required time-frame, for instance in the operating room or the trauma bay for longer than 60 minutes.5
Storage and transportation
Proper storage and transportation of blood components are critical for safe transfusion. If improperly stored, blood, as a biological product, carries the risk of bacterial contamination. Improper storage may also affect blood component efficacy.
The shelf life of a red blood cell unit issued by Canadian Blood Services is 42 days from collection. Manipulation of the unit, including washing or irradiation, shortens the shelf life. The expiry date is documented on the label of each unit (see Figure 2). If the red blood cell unit’s bag is breached without the use of a sterile connection device, the shelf life is limited to 24 hours if stored at 1–6°C (or the original expiry date, whichever is sooner), or to 4 hours if stored above 6°C.6 Red blood cell components may be irradiated up to 28 days after collection. Following irradiation, irradiated cells must be transfused as soon as possible, but not later than 14 days after irradiation and no later than 28 days after collection, whichever comes first.7
Red blood cell components must be stored at 1–6°C in a temperature-controlled storage device with an alarm system, air-circulating fan and continuous monitoring device.4 During storage and transportation, records must be kept to maintain a chain of traceability and to ensure that appropriate conditions were present throughout this time frame4.
Maintaining proper storage temperature during transportation is essential. Transportation time for red blood cells should not exceed 24 hours (not including time needed to issue and pack), using Canadian Blood Services validated shipping containers and standardized packing procedures8 to maintain an environmental temperature of 1–6°C. For transit times of 24 hours or less, a transport system validated to maintain an environmental temperature of 1–10°C is allowable.4 Visual inspection of each blood component to be shipped must be performed and documented at the time of shipping and receiving.
When red blood cell units accompany a patient who is transferred from one facility to another, traceability of the red blood cell unit must be maintained. Accordingly, the prescribing hospital transfusion service is responsible for appropriate issuing and the receiving hospital transfusion service is responsible for the final disposition documentation (transfused vs. discarded).6 It is the collective responsibility of both sites to ensure any adverse events are investigated and appropriately managed.6
Further details about red blood cell units processed by Canadian Blood Services can be found in the Circular of Information for Red Blood Cells, Leukocytes Reduced.
Available alternatives
Depending on the underlying cause of anemia, alternative treatments that may be considered include oral iron, intravenous iron, vitamin B12, folic acid and/or erythropoietin stimulating agents. Monitoring the patient while treating the underlying condition(s) contributing to anemia may be an alternative to transfusion for some patients.3
Whole blood, leukocytes reduced
Component production and description
In October 2022, Canadian Blood Services received Health Canada approval to process and distribute whole blood, leukocytes reduced (LrWB). The component was initially available only for military use, but this restriction was removed following recommendations from the National Advisory Committee on Blood and Blood Products (NAC).9 In January 2025, Canadian Blood Services began producing and distributing LrWB to hospitals participating in clinical studies of LrWB (see Canadian Blood Services’ customer letter and additional criteria).
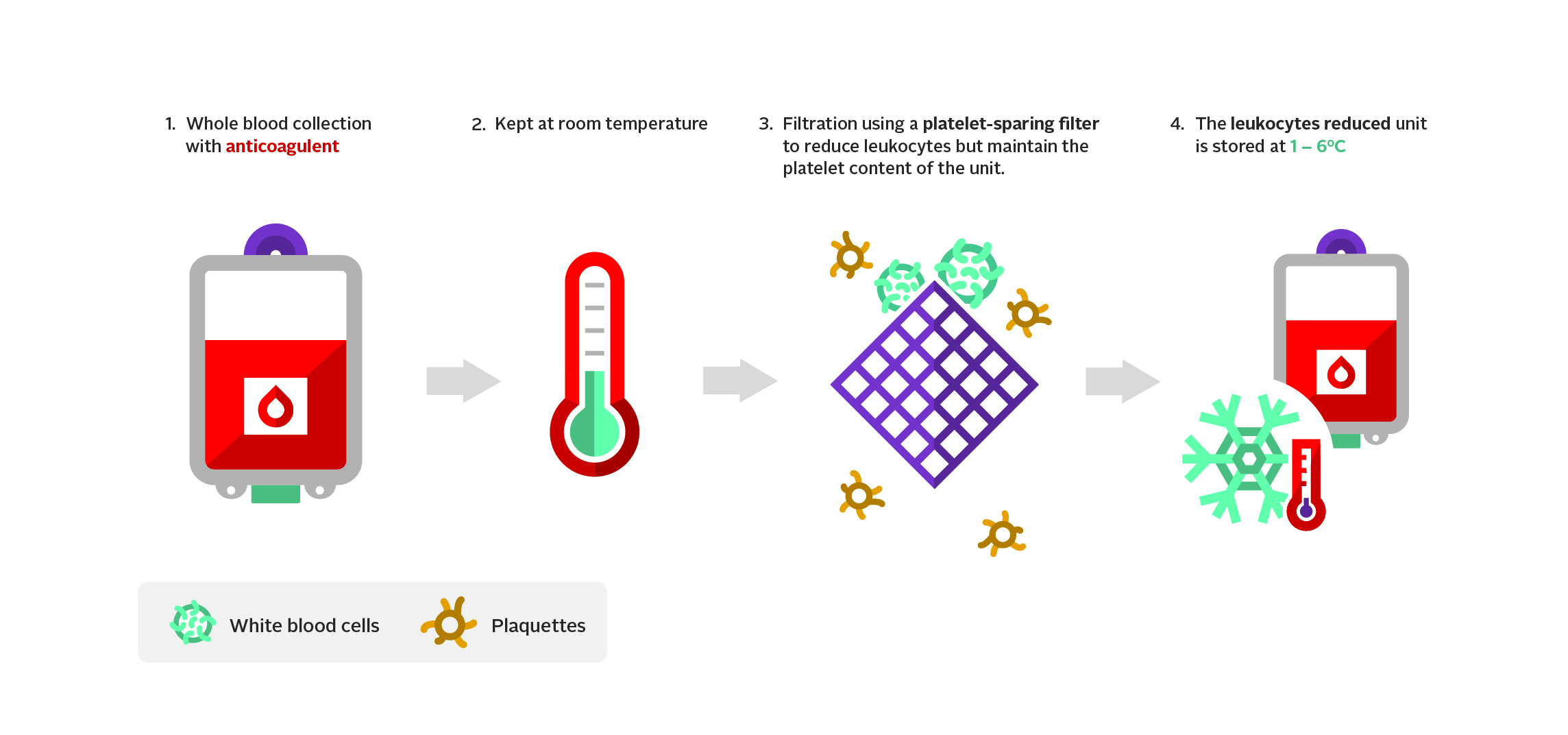
LrWB is produced from a whole blood donation, where approximately 480 mL of whole blood is collected from the donor into a collection system containing 70 mL of citrate-phosphate-dextrose (CPD) anticoagulant. LrWB units are produced from blood donations from male, group O donors, either RhD-positive or –negative, with low-titres of anti-A/B isohemagglutinins. Only blood donations with a titre less than 1:128 by manual immediate spin equivalent are used for LrWB production. The use of low-titre anti-A/B, group O blood donations to produce LrWB reduces the risk of severe hemolytic transfusion reactions but does not eliminate the risk entirely. More information can be found in our FAQ: Donor high titre isohemagglutinin (anti-A/anti-B) testing at Canadian Blood Services.
Once the donation is collected, it is kept at room-temperature for a minimum of 4 hours, then the bag of anti-coagulated whole blood is connected to a whole blood filter system for gravity filtration. This system uses a platelet-sparing filter to reduce the number of leukocytes while preserving the platelet concentration. The filtered unit is stored at 1–6˚C within 24 hours of the end of collection (see Figure 3). A typical LrWB unit issued by Canadian Blood Services is 496 mL, contains 62 g of hemoglobin, 234 mL of plasma, has a hematocrit of approximately 41%, and an average residual leukocyte count of 0.2 x 106. For more information, refer to the Circular of Information, Whole Blood, Leukocytes Reduced and the publication FAQ: Whole blood, leukocytes reduced (LrWB).
Indications
LrWB is indicated to treat clinically significant bleeding. Each unit of LrWB provides red blood cells, plasma, and platelets in physiological ratios. Transfusion of one unit of LrWB is similar to providing one unit of red blood cells, one unit of frozen plasma, and one fourth of a unit of platelet concentrate, with much less citrate. The clinical use of LrWB remains an area of active research.
Contraindications
The clinical use of LrWB is an area of active research, as such, contraindications for its use are also under investigation.
Dose and administration
In adults, the dosing for LrWB is based on the clinical assessment of the patient with bleeding. The volume of group O LrWB that can safely be administered to a non-O patient is not well defined, limits of 6–8 units of low-titer group O LrWB are used by some adult centers.10, 11 For pediatric use, the suggested dosing is 20–40 mL per kg.12 Patients with ongoing bleeding may require higher doses, but administration should be guided by laboratory testing to monitor hematologic response and electrolyte disturbances. Group O LrWB has been safely administered to patients of unknown blood group. The plasma in each unit of group O LrWB contains anti-A and anti-B isohemagglutinins.
For administration, a standard set containing a 170–260 micron or equivalent filter must be used for infusion. A blood warmer licensed by Health Canada for that purpose may be used at the discretion of the recipient’s physician. ABO-compatible blood products, 5% albumin, or 0.9% sodium chloride injection, can be co-administered at the discretion of the recipient’s physician.
Transfusion rate is determined based on clinical assessment of the patient. As with other component use, recipients need to be under clinical observation, with close observation during the first 15 minutes.
Storage and transportation
LrWB units produced by Canadian Blood Services should be stored at 1–6˚C without agitation. LrWB collected in CPD expires after 21 days, unless otherwise specified. Once punctured, units of LrWB should be transfused within 4 hours if stored at >6˚C, and within 24 hours if stored at 1–6˚C.
For more information on LrWB, please read the Circular for Information.
Available alternatives
In a bleeding patient, transfusion may include use of red blood cells, platelets, plasma, solvent detergent treated plasma, fibrinogen concentrate, or cryoprecipitate as well as life-saving adjuncts such as tranexamic acid. For a detailed discussion on management of patients with massive hemorrhage or coagulopathy of trauma, please read Chapter 11 of this Guide.
Platelets
Component production and description
Canadian Blood Services produces a number of platelet types (see Chapter 19 for details of the manufacturing processes):
- Pooled platelet psoralen-treated (PPPT) are produced from whole blood collected into a buffy coat collection set with CPD anticoagulant (Figure 1).
- Apheresis platelet psoralen-treated (APPT) are produced from a single-donor apheresis platelet donation.
- Untreated apheresis platelet in PAS-E is similar to APPT production, except no pathogen inactivation occurs.
Platelets are labeled as Rh negative only when all contributing units within the pooled component are Rh negative. The pool is labeled as “low anti-A/B” only when all the units contributing to the pool were found to have anti-A and anti-B levels below a predetermined cut-off (see the FAQ: Donor high titre isohemagglutinin (anti-A, anti-B) testing at Canadian Blood Services).
Platelets are produced within 28 hours of collection, combining transportation and manufacturing time. The pathogen inactivation process for platelets is then completed by midnight the day after collection and a maximum of 16 hours later, the treated platelet is transferred to the final storage container. Please see Chapter 19 of this Guide for more details on pathogen inactivation and the applicable Circular of Information for further characteristics of platelet components.
Indications
The transfusion of platelets is indicated in the treatment of patients with bleeding due to severely decreased or dysfunctional platelets. Platelet transfusion may also be useful if given prophylactically to patients with rapidly falling or low platelet counts secondary to bone marrow disorders or chemotherapy.13 See Chapter 18 of this Guide for more information on therapeutic and prophylactic platelet transfusions.
Indications are similar for both pooled platelets and apheresis platelets. Apheresis platelets may be selected based on the human leukocyte antigen (HLA) profile when a recipient fails to respond to platelet transfusion because of demonstrated anti-HLA antibodies (alloimmune refractoriness). See Chapter 18 of this Guide for details on treatment and testing for platelet-refractory patients.
Contraindications
Platelet transfusions are not recommended for patients with immune thrombocytopenic purpura (ITP), heparin-induced thrombocytopenia (HIT) or thrombotic thrombocytopenic purpura (TTP) and platelet transfusion may cause worsening of these conditions or cause increased thrombosis risk.
Dose and administration
While ABO-identical platelets may be preferred for transfusion of some patients, ABO compatible platelets are often used. See Chapter 9 and Chapter 18 of this Guide for information on ABO compatibility, dose and administration of platelet components.
Transfusion of apheresis platelets should result in increments like those achieved by transfusion of pooled platelets. Each dose of platelets should increase the patient’s platelet count at 1 hour by 15–25 x 109/L in a 70 kg adult.14 Sepsis, alloimmunization, fever, ITP, or disseminated intravascular coagulation (DIC) may contribute to a lower post-transfusion increment. See Chapter 18 of this Guide for more information.
Storage and transportation
Platelet components must be stored at 20–24°C with continuous gentle agitation. If the agitator is not a closed platelet incubator, the ambient temperature must be recorded manually using a calibrated thermometer every four hours or through use of a constant room temperature monitoring device.4
In Canada, pooled platelets psoralen-treated, apheresis platelets psoralen-treated and untreated apheresis platelets PAS-added have a shelf life of seven days from the date of collection. Once breached, the unit expiry time is four hours. Aliquots may be prepared using sterile connection devices (e.g., in the case of pediatric patient use). Aliquots obtained using sterile connection devices retain the original seven-day expiry date and must contain a minimum residual volume. For psoralen-treated platelets (both apheresis and pooled) the minimal residual volume is 135ml. For untreated apheresis platelets PAS added the minimum residual volume is 100ml. The collection and expiry dates indicated on the platelet unit must be copied to the label of each aliquot pack made from the original unit.
Further details on the platelet components produced by Canadian Blood Services can be found in the applicable Circular of Information, Chapter 18 and Chapter 19 of this Guide.
Available alternatives
Apheresis platelets may be substituted for pooled platelets if supply and demand allow.
Other hemostatic strategies may be considered as an alternative to platelet concentrates, such as tranexamic acid or factor concentrates.
Plasma components
Component production and description
Canadian Blood Services produces and distributes the following types of plasma components:
- Apheresis Frozen Plasma in ACD-A
- Apheresis frozen plasma, psoralen treated in ACD-A (AFP-PT)
- Frozen plasma CPD (FP)
- Cryoprecipitate (see cryoprecipitate section later in this chapter)
Canadian Blood Services also provides:
- Octaplasma (Solvent detergent treated (S/D) plasma) produced by Octapharma.
Blood donors are screened for the presence of antibodies to red cell antigens. If a clinically significant antibody is identified, the plasma is discarded.
FP is prepared from whole blood collected in CPD anticoagulant that is red blood cell- and platelet-reduced by centrifugation. The extracted plasma is frozen within 24 hours of collection and labelled as a frozen plasma CPD unit. FP is not labelled as leukoreduced but the processing steps result in a significant reduction in the number of leukocytes present.6
For pediatric patients requiring small volume transfusions, Canadian Blood Services offers divided plasma units (FP-divided) which contain 125–150 mL of plasma. FP-divided is blood group AB, unless otherwise requested.
The typical volume of FP issued by Canadian Blood Services and their coagulation factor content are described in Table 1. Coagulation factors V and VIII are labile coagulation factors and are not stable in plasma stored for prolonged periods at 1–6˚C; therefore, plasma is stored frozen at -18˚C or lower. FP contains factor VIII levels that are approximately 70–75% of the levels present at the time of collection, and according to Canadian standards must contain at least 0.52 IU/mL of factor VIII, in at least 75% of the units tested. The levels of factor V and other coagulation factors are not significantly decreased from baseline in plasma frozen within 24 hours of collection.
Canadian Blood Services is currently in the process of adding apheresis frozen plasma, psoralen-treated (AFP-PT) in ACD-A to its component list with a planned implementation date of the Fall of 2025 (see the customer letter CL_2025-26 on blood.ca for more information). More information on this component can be found in a separate publication entitled, Apheresis frozen plasma, psoralen-treated.
Canadian Blood Services also supplies Octaplasma. This is a S/D treated, pooled fresh frozen plasma component processed by Octapharma (for more information about Octapharma, see our publication Solvent detergent (S/D) treated plasma (Octaplasma) and the Octaplasma product monograph).15 Octaplasma is filtered to remove cells and debris, which may help reduce adverse events linked to the presence of residual blood cells, and the pooling process dilutes and neutralizes allergens and antibodies, theoretically reducing the risk of transfusion-related acute lung injury (TRALI). The manufacturing process with S/D treatment destroys enveloped viruses and resin adsorption removes prions, reducing but not eliminating the risk of infection and variant-Creutzfeldt Jakob disease (vCJD).15
Table 1. Description of plasma components distributed by Canadian Blood Services
| Type | Description |
|---|---|
| Apheresis Frozen Plasma (AFP in ACD-A) | Approximately 249 mL of plasma collected through plasmapheresis. Contains approximately 38 mL of ACD-A anticoagulant, stored in a ≤ -18°C freezer within 24 hours of collection. On average, it contains 1.05 IU of Factor VIII per mL. |
| Frozen Plasma CPD (FP) | Approximately 289 mL of plasma separated from an individual unit of whole blood collected in CPD anticoagulant and placed in a freezer at ≤ -18˚C within 24 hours after collection; contains all coagulation factors but has slightly reduced amounts of clotting Factors V and VIII. On average, FP contains 0.88 IU of Factor VIII per mL. |
| Apheresis plasma, psoralen-treated (AFP-PT) | Approximately 203 mL of plasma collected through plasmapheresis in ACD-A anticoagulant. After collection, the component undergoes further processing within 24 hours and is stored in a ≤-18°C freezer. On average, it contains 1.17 IU of Factor VIII per mL. See the publication, Apheresis frozen plasma, psoralen-treated for more information. |
| Octaplasma, Solvent Detergent (S/D) Plasma | Plasma pooled from many donors, treated with processing steps (solvent detergent, immune neutralization, sterile filtration) to remove or inactivate pathogens, cells, allergens and antibodies. Each unit is 200 mL of cell-free plasma that contains 9.0–14.0 g of human plasma proteins (45–70 mg/mL). A minimum of 0.5 IU per mL is obtained for all clotting factors. |
Indications
The use of plasma is limited almost exclusively to the treatment or prevention of clinically significant bleeding due to a deficiency of one or more coagulation factors for which more appropriate or specific alternative therapy is not available. Such situations potentially include the treatment of:
- Bleeding patients or patients undergoing invasive procedures who require replacement of multiple coagulation factors (such as patients with severe liver disease or disseminated intravascular coagulation)
- Patients with massive hemorrhage with clinically significant coagulation abnormalitie
- Patients on warfarin anticoagulation who are bleeding or undergoing an invasive procedure before vitamin K can reverse the warfarin effect and for whom prothrombin complex concentrates (PCC) are contraindicated (i.e., history of HIT) or unavailable.
- Patients requiring treatment of TTP by plasma exchange
- Other conditions treated by therapeutic plasma exchange where the exchange fluid must include coagulation factors. See Chapter 14 of this Guide.
FP or S/D plasma may also be used in the preparation of reconstituted whole blood. See Chapter 13 of this Guide for more information about neonatal and pediatric plasma transfusion.
FP is one of the most misused blood components and efforts are being made to promote appropriate dosing and utilization.16
Octaplasma is available and approved for use in Canada for the same indications as FP. See the National Advisory Committee on Blood and Blood Products (NAC) Recommendations for the use of solvent-detergent plasma in Canada.
See also Chapter 17 of this Guide and the Canadian Blood Services Circular of Information, Plasma Components.
Contraindications
Plasma transfusion is not indicated for volume replacement alone, or for coagulation factor deficiency if specific recombinant products or plasma-derived virally inactivated products are available. Plasma transfusion is generally not indicated or effective in a non-bleeding patient for primary prophylaxis when the International Normalized Ratio (INR) is below 1.8.
Hypovolemia without coagulation factor deficiencies should be treated with isotonic crystalloids (normal saline or Ringer’s lactate solution).
Do not use plasma when coagulopathy can be more appropriately corrected with specific therapy such as vitamin K, PCC, cryoprecipitate, or specific coagulation factor replacement. See Chapter 5 of this Guide for information about coagulation factor concentrates available in Canada and their use.
Plasma should not be used in the reversal of direct oral anticoagulants (DOACs).
Dose and administration
Chapter 9 and Chapter 13 of this Guide provide detailed information on blood administration and neonatal and pediatric transfusion, respectively.
The volume transfused depends on the clinical situation and recipient size, and when possible, should be guided by serial laboratory assays of coagulation function. The dose of FP to achieve a minimum of 30% of plasma clotting factor concentration is attained with administration of 10–15 mL/kg of body weight, approximately 3–4 units in an adult. The product monograph of Octaplasma suggests that 12–15 mL/kg is a generally accepted starting dose, depending on the clinical situation and underlying disorder and that an adequate hemostatic effect on minor or moderate hemorrhages or surgery is normally achieved after the infusion of 5–20 mL/kg of Octaplasma.15
Plasma components must be ABO compatible with the recipient but are not necessarily required to be group specific. In most clinical circumstances, the plasma component should not contain ABO antibodies that may be incompatible with the ABO antigens on the patient’s red blood cells. If there is no ABO group available for the recipient, typing will be required to determine compatibility. For the initial management of massive hemorrhages, group AB plasma is predominantly used in Canada, although many North American trauma centres opt to use group A plasma as an unmatched alternative. At Canadian Blood Services, all blood donations are tested for isohemagglutinin titres and over 90% of group A plasma is labelled as low titre (indicated by “low anti-A/B” on the label) which facilitates its use in this context.
Thawing of FP may take 12–30 minutes depending on the component volume, thawing method and equipment used by the hospital transfusion service. S/D plasma (Octaplasma) should be thawed in the outer wrapper in a circulating water bath (30–37°C) for 30 to 60 minutes or in a dry tempering system according to product instructions. Detailed device-specific recommendations are available from Octapharma.15 Upon completion of thawing, plasma components should be transfused immediately or stored in a continuously temperature-monitored refrigerator at 2–8°C for up to five days. Once thawed, plasma components cannot be refrozen.
If the transfusion of the plasma unit will not be initiated promptly after removal from the temperature-controlled storage or transportation device, it should be returned immediately to inventory to prevent waste. Blood components may be returned only if the bag is intact, passes a visual inspection and has maintained an acceptable temperature (See CSTM Standard 5.8.7.2 for more information).
Storage and transportation
Frozen plasma components must be stored frozen at -18°C or colder in a controlled, temperature-monitored freezer for a maximum of 12 months (FP, AFP in ACD-A) or four years (Octaplasma). Plasma components must not be out of a temperature-controlled environment for longer than 30 minutes.6
Available alternatives
CBS processed plasma components and S/D plasma may be used interchangeably depending on indication, supply and demand.
Vitamin K should be used for warfarin reversal when the patient is not bleeding or does not require an invasive procedure urgently. Patients requiring rapid reversal of warfarin due to bleeding, bleeding risk or an urgent invasive procedure may benefit from use of a PCC along with Vitamin K. Practical guidance on warfarin reversal can be found on Treat the Bleed, an evidence-based, online resource. The Thrombosis Canada website also provides practical resources, including a clinical tool for bleed management.
Specific plasma protein concentrates are available and are described in Chapter 5 and Chapter 17 of this Guide.
Cryoprecipitate
Component production and description
Canadian Blood Services prepares cryoprecipitate from available frozen plasma (FP) that is centrifuged to separate the insoluble cryoprecipitate from the residual plasma (cryosupernatant). The latter is removed, and the insoluble component is refrozen and labelled as cryoprecipitate. Typically, one unit of cryoprecipitate (10 ± 2 mL) is obtained from one unit of FP. A typical unit of cryoprecipitate contains approximately 366 mg of fibrinogen.
As of March 31, 2025, Canadian Blood Services will discontinue production of cryosupernatant, see the customer letter for further details. This product will be available until the inventory is depleted, which is estimated to occur by November 2025.
Indications
Over the last several years, the clinical indications for fibrinogen replacement and role of cryoprecipitate have changed because of a better understanding of how to manage coagulopathy, greater recognition of the non-factor VIII constituents within cryoprecipitate, preference for products that have undergone viral inactivation, and the development of alternative factor concentrates
Cryoprecipitate has traditionally been used for fibrinogen replacement in acquired hypofibrinogenemia in a bleeding patient, but increasingly in Canada, fibrinogen concentrate is being used in its place. While both are plasma-derived and acceptable options for fibrinogen replacement therapy, there are several advantages to using fibrinogen concentrate. Fibrinogen concentrate has been purified, pathogen reduced and has a standardized fibrinogen content. Available as a lyophilized powder it can be easily stored, reconstituted and administered with a longer shelf life after reconstitution to minimize wastage. By contrast, cryoprecipitate is a non-purified component that contains other clotting factors. It is shipped and stored frozen and must be thawed prior to use, which once thawed, can be slow and cumbersome to administer. The relatively short shelf-life after thawing (4 hours) increases the risk of wastage. For these reasons, fibrinogen concentrate is increasingly used in Canada for the treatment of acquired hypofibrinogenemia.17, 18
Generally, the combination of clinically significant bleeding and a plasma fibrinogen level of less than 1.0 g/L, provides an objective basis for fibrinogen replacement. These situations include individuals with acquired hypofibrinogenemia and bleeding in the context of disseminated intravascular coagulation. Certain populations have a higher threshold (e.g., fibrinogen <2.0 g/L in massively bleeding obstetrical patients and cardiac surgery-related bleeding; fibrinogen <1.5 g/L for non-obstetric patients with massive hemorrhage). A recent study in cardiac surgery related bleeding confirms the efficacy of fibrinogen concentrate in this setting.18 The only clinical situation where fibrinogen replacement is routinely used as primary prophylaxis is in the early management of acquired hypofibrinogenemia associated with acute promyelocytic leukemia4, 17, 19, 20
The historical use of cryoprecipitate as a factor VIII and vWF concentrate for hemophilia and von Willebrand disease has now been replaced with recombinant clotting factor concentrates
Contraindications
Specific factor and/or recombinant concentrates are preferred, when available, because of the reduced risk of transfusion-transmissible diseases and the provision of more predictable and consistent dosing. Cryoprecipitate is not recommended in the treatment of hemophilia A or von Willebrand disease as safer purified concentrates and recombinant products are available; see Chapter 5 and Chapter 7 of this Guide.
Fibrinogen concentrates may be preferred for the treatment of hypofibrinogenemia or dysfibrinogenemia. Cryoprecipitate should not be used to make fibrin glue; virally inactivated commercial products should be used for this purpose.
Dose and administration
Group-specific cryoprecipitate is not necessary outside of the pediatric setting and should be considered according to local policy for pediatric recipients. Chapter 9 and Chapter 13 of this Guide provided detailed information on blood administration and on neonatal and pediatric transfusion, respectively.
One unit of cryoprecipitate contains approximately 366 mg of fibrinogen. The amount of cryoprecipitate required will depend on the severity and nature of the bleeding condition. While the amount of cryoprecipitate needed to raise the fibrinogen concentration of plasma can be calculated (see Figure 4), it is common practice to use a generic dose of one cryoprecipitate unit per 10 kg body weight as a first dose, with additional doses as required to maintain the fibrinogen level for the clinical scenario. For example, an order of 10 units of cryoprecipitate for a 70kg adult patient will provide approximately 3 to 4 g of fibrinogen.
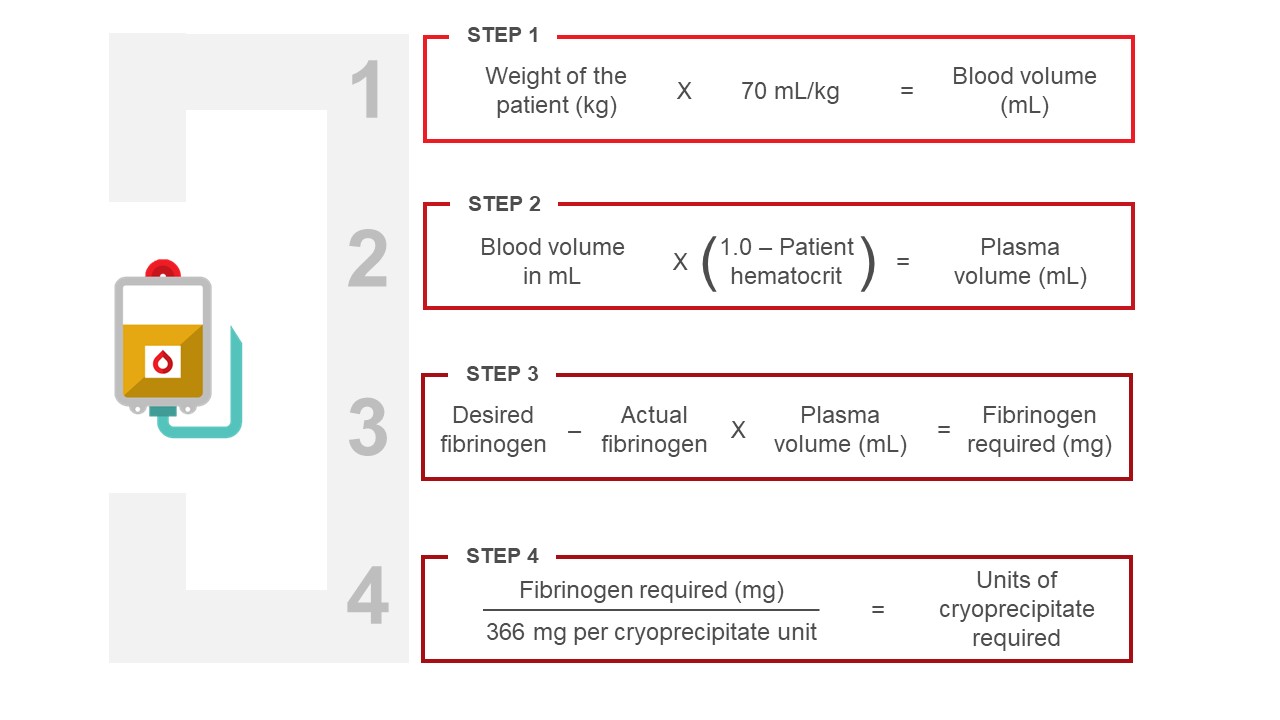
Cryoprecipitate is often pooled by hospital transfusion medicine service personnel or may be given as individual units sequentially. Small quantities of normal saline are introduced to rinse each bag in the pooling process. If the pooled cryoprecipitate is assigned a new product code by the hospital transfusion medicine laboratory, the new label must indicate the number of units in the pool. For traceability, either the new product code or every contributing donor unit number must be directly recorded in the medical record of the recipient. Thawed cryoprecipitate must be used within four hours.
Cryoprecipitate may be administered through a blood administration set with a standard blood filter or as a filtered bolus injection by trained personnel. Infusion should be as rapid as can be tolerated by the patient or as specified by the ordering physician.
Storage and transportation
Cryoprecipitate must be stored frozen at -18°C or colder in a temperature controlled, monitored freezer for a maximum of 12 months. Components must not be out of the temperature-controlled blood storage freezer for longer than 30 minutes. If the transfusion will not be initiated promptly after removal from the temperature-controlled blood component storage device, the component should be returned immediately to prevent deterioration and waste.4 Once thawed, cryoprecipitate cannot be refrozen and should be stored at 20–24°C and transfused within four hours.
More information about cryoprecipitate can be found in the Circular of Information for the Use of Human Blood Components, Plasma Components.
Available alternatives
Clotting factor concentrates are preferred for clotting factor FVIII, vWF, and FXIII and fibrinogen replacement; although, cryoprecipitate may be considered as an alternative if these agents are not available. See Chapter 5 of this Guide for information on coagulation factor concentrates available in Canada and their use. See also Chapter 17 of this Guide for information on treating hemostatic disorders including hemophilia and von Willebrand disease.
While cryoprecipitate contains FXIII, the preferred product for management of FXIII deficiency is FXIII concentrates. 21, 22 Cryoprecipitate may be considered as an alternative if FXIII replacement is urgently needed and Factor XIII concentrate is not readily available.
Continuing professional development credits
Fellows and health-care professionals who participate in the Canadian Royal College's Maintenance of Certification (MOC) program can claim the reading of the Clinical Guide to Transfusion as a continuing professional development activity under Section 2: Individual learning. Learners can claim 0.5 credits per hour of reading to a maximum of 30 credits per year.
Medical laboratory technologists who participate in the Canadian Society for Medical Laboratory Sciences’ Professional Enhancement Program (PEP) can claim the reading of the Clinical Guide to Transfusion as a non-verified activity.
Suggested citation
Rotin L, Mack J. Blood components. In: Khandelwal, A, Abe T, editors. Clinical Guide to Transfusion [Internet]. Ottawa: Canadian Blood Services, 2025 [cited YYYY MM DD]. Chapter 2. Available from: https://professionaleducation.blood.ca
Acknowledgements
The authors would like to acknowledge the contributions of Akash Gupta, MD, Mark Bigham, MD, MHSc and Gwen Clarke, MD for their contributions to previous versions of this chapter. The authors would also like to acknowledge the subject matter expert reviews of Melanie Bodnar, MD, Asim Alam, MD and Akash Gupta, MD for the current version of this chapter.
If you have questions about the Clinical Guide to Transfusion or suggestions for improvement, please contact us through the Clinical Guide feedback form.
References
1. Carson, J. L., Stanworth, S. J., Guyatt, G., Valentine, S., Dennis, J., Bakhtary, S., Cohn, C. S., Dubon, A., Grossman, B. J., Gupta, G. K., Hess, A. S., Jacobson, J. L., Kaplan, L. J., Lin, Y., Metcalf, R. A., Murphy, C. H., Pavenski, K., Prochaska, M. T., Raval, J. S., . . . Pagano, M. B. (2023). Red Blood Cell Transfusion: 2023 AABB International Guidelines. JAMA, 330(19), 1892-1902. https://doi.org/10.1001/jama.2023.12914
2. Vlaar, A. P., Oczkowski, S., de Bruin, S., Wijnberge, M., Antonelli, M., Aubron, C., Aries, P., Duranteau, J., Juffermans, N. P., Meier, J., Murphy, G. J., Abbasciano, R., Muller, M., Shah, A., Perner, A., Rygaard, S., Walsh, T. S., Guyatt, G., Dionne, J. C., & Cecconi, M. (2020). Transfusion strategies in non-bleeding critically ill adults: a clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Med, 46(4), 673-696. https://doi.org/10.1007/s00134-019-05884-8
3. Canadian Society for Transfusion Medicine. (2017). Transfusion Medicine Recommendations. Choosing Wisely Canada. https://choosingwiselycanada.org/transfusion-medicine/
4. CSTM Standards Committee. (2022). CSTM Standards for Hospital Transfusion Services (5 ed.). Canadian Society for Transfusion Medicine.
5. Accreditation Canada. (2023). Accreditation Canada Diagnostics, Medical Laboratory Accreditation Requirements Version 9. In (9 ed.). Ontario: Accreditation Canada.
6. Canadian Standards Association (CSA) Group. (2020). CAN/CSA-Z902-20 Blood and Blood Components (4th ed.). CSA Group. https://www.csagroup.org/store/product/2427533/ (2004)
7. National Advisory Committee on Blood and Blood Products. (2023). Recommendations for use of irradiated blood components in Canada: A NAC and CCNMT collaborative initiative. https://nacblood.ca/en/guidelines-recommendations
8. Ludwig, C. (2015). Thermal Qualification of Series 4-8L Insulated Shipping Containers for RBC Transport.
9. Pambrun, C., Tinmouth, A., Shih, A., Morrison, D., Pavenski, K., Nahirniak, S., Petraszko, T., Simpson, C., Eduljee, C., Mack, J., Dawson, R., Beckett, A., Lang, E., Stoffman, J., Bartoszko, J., & Robitaille, N. (2024). Whole Blood, Leukocytes Reduced Recommendations. Guidelines and Recommendations. https://nacblood.ca/en/resource/whole-blood-leukocytes-reduced-recommendations
10. Harrold, I. M., Seheult, J. N., Alarcon, L. H., Corcos, A., Sperry, J. L., Triulzi, D. J., & Yazer, M. H. (2020). Hemolytic markers following the transfusion of uncrossmatched, cold-stored, low-titer, group O+ whole blood in civilian trauma patients. Transfusion, 60 Suppl 3, S24-s30. https://doi.org/10.1111/trf.15629
11. Yazer, M. H., Corcos, A., J, L. S., Triulzi, D. J., & Leeper, C. (2022). Receipt of at least 4 units of low titer group O whole blood with titer <100 does not lead to hemolysis in adult trauma patients. Transfusion, 62 Suppl 1, S72-s79. https://doi.org/10.1111/trf.16980
12. Abou Khalil, E., Morgan, K. M., Gaines, B. A., Spinella, P. C., & Leeper, C. M. (2024). Use of whole blood in pediatric trauma: a narrative review. Trauma Surg Acute Care Open, 9(Suppl 1), e001127. https://doi.org/10.1136/tsaco-2023-001127
13. Nahirniak, S., Slichter, S. J., Tanael, S., Rebulla, P., Pavenski, K., Vassallo, R., Fung, M., Duquesnoy, R., Saw, C. L., Stanworth, S., Tinmouth, A., Hume, H., Ponnampalam, A., Moltzan, C., Berry, B., & Shehata, N. (2015). Guidance on platelet transfusion for patients with hypoproliferative thrombocytopenia. Transfus Med Rev, 29(1), 3-13. https://doi.org/10.1016/j.tmrv.2014.11.004
14. Kaufman, R. M., Djulbegovic, B., Gernsheimer, T., Kleinman, S., Tinmouth, A. T., Capocelli, K. E., Cipolle, M. D., Cohn, C. S., Fung, M. K., Grossman, B. J., Mintz, P. D., O'Malley, B. A., Sesok-Pizzini, D. A., Shander, A., Stack, G. E., Webert, K. E., Weinstein, R., Welch, B. G., Whitman, G. J., . . . AABB. (2015). Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med, 162(3), 205-213. https://doi.org/10.7326/M14-1589
15. Octapharma Canada. (2022). Octaplasma Product Monograph, October 31, 2022. https://www.octapharma.ca/en/therapies/product-overview
16. Khandelwal, A., Minuk, L., Liu, Y., Arnold, D. M., Heddle, N. M., Barty, R., Hsia, C., Solh, Z., Shehata, N., Thompson, T., Tinmouth, A., Perelman, I., Skeate, R., Kron, A. T., & Callum, J. (2022). Plasma transfusion practices: A multicentre electronic audit. Vox Sang, 117(10), 1211-1219. https://doi.org/10.1111/vox.13355
17. National Advisory Committee on Blood and Blood Products. NAC Statement on Fibrinogen Concentrate, 2018. https://www.nacblood.ca/resources/guidelines/downloads/NAC_fibrinogen_concentrate_FINAL.pdf
18. Callum, J., Farkouh, M. E., Scales, D. C., Heddle, N. M., Crowther, M., Rao, V., Hucke, H. P., Carroll, J., Grewal, D., Brar, S., Bussières, J., Grocott, H., Harle, C., Pavenski, K., Rochon, A., Saha, T., Shepherd, L., Syed, S., Tran, D., . . . Karkouti, K. (2019). Effect of Fibrinogen Concentrate vs Cryoprecipitate on Blood Component Transfusion After Cardiac Surgery: The FIBRES Randomized Clinical Trial. JAMA, 322(20), 1966-1976. https://doi.org/10.1001/jama.2019.17312
19. Spahn, D. R., Bouillon, B., Cerny, V., Duranteau, J., Filipescu, D., Hunt, B. J., Komadina, R., Maegele, M., Nardi, G., Riddez, L., Samama, C.-M., Vincent, J.-L., & Rossaint, R. (2019). The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care, 23(1), 98. https://doi.org/10.1186/s13054-019-2347-3
20. Stein, E., McMahon, B., Kwaan, H., Altman, J. K., Frankfurt, O., & Tallman, M. S. (2009). The coagulopathy of acute promyelocytic leukaemia revisited. Best Pract Res Clin Haematol, 22(1), 153-163. https://doi.org/10.1016/j.beha.2008.12.007
21. Alshehri, F. S. M., Whyte, C. S., & Mutch, N. J. (2021). Factor XIII-A: An Indispensable "Factor" in Haemostasis and Wound Healing. Int J Mol Sci, 22(6). https://doi.org/10.3390/ijms22063055
22. Kleber, C., Sablotzki, A., Casu, S., Olivieri, M., Thoms, K. M., Horter, J., Schmitt, F. C. F., Birschmann, I., Fries, D., Maegele, M., Schöchl, H., & Wilhelmi, M. (2022). The impact of acquired coagulation factor XIII deficiency in traumatic bleeding and wound healing. Crit Care, 26(1), 69. https://doi.org/10.1186/s13054-022-03940-2