Platelet product quality remains high after seven days of storage
Authors: Katherine Serrano, PhD, Peter Schubert, PhD, and Dana Devine, PhD
Note: This article is relevant for platelet units manufactured from buffy coat or collected by apheresis. It does not apply to pathogen-reduced platelets, also known as pooled platelets psoralen-treated (PPPT); more information on PPPT is available in the Canadian Blood Services’ publication, Pathogen-reduced buffy coat platelets.
Background
In August 2017, Canadian Blood Services changed its platelet product testing procedure to improve the detection of bacterial contamination. This change enhanced the safety of Canadian Blood Services platelet products and provided the opportunity to improve inventory management by extending the platelet shelf life from five to seven days.
Many biological processes continue in platelets during storage, which raises an important question: do the extra two days of storage significantly alter platelet quality? To find out, our researchers examined in vitro quality parameters of platelets. Buffy coat platelet concentrates were stored under standard conditions (20–24°C, with gentle agitation) until analysis at day five and day seven of storage. Apheresis platelets were also examined and showed similar trends (data not shown). For details on Canadian Blood Services platelet production methods, see the Blood Components chapter of our Clinical Guide to Transfusion.
The researchers assessed quality by looking at platelet concentration, volume, metabolism, and markers of platelet activation and responsiveness.
Platelet concentration, volume and metabolism
Platelet concentration measures the number of platelets in the storage bag. Glucose, lactate, and pH reflect metabolism of platelets in the bag. When platelets in a storage bag are metabolically active, glucose would be expected to decrease as it is consumed during metabolism, while lactate increases as it is produced.
Table 1: Concentration, volume and metabolic markers of buffy coat platelets at day five versus day seven of storage.
| Quality parameter | Day 5 | Day 7 | n | |
|---|---|---|---|---|
| Cellular |
Platelet concentration (x 109/L) |
821 ± 79 | 825 ± 109 | 26 |
| Platelet size (fL) | 8.6 ± 0.3 | 8.6 ± 0.3 | 13 | |
| Metabolism |
Glucose (mM) |
14.6 ± 1.4 | 13.4 ± 1.3* | 26 |
| Lactate (mM) | 12.2 ± 0.8 |
15.7 ± 1.3* |
26 | |
| pH | 7.24 ± 0.07 | 7.19 ± 0.08* | 26 | |
|
Table shows mean values ± standard deviation. * p < 0.05, day five versus day seven, paired Student’s t test. |
Key observations
- The concentration of platelets in the storage bag and the average platelet volume remained constant over the two additional days of storage.
- Glucose levels and pH decreased between days five and seven whereas lactate levels increased.
Platelet activation and responsiveness
The longer platelets are stored, the more activated they become, so it was important to assess the extent of platelet activation in platelets stored for seven versus five days. The researchers used several measures to assess this. CD62P is a marker for degranulation, which is associated with platelet activation; CD62P positivity correlates with the percentage of platelets that have become activated. Platelets also change shape upon activation, from smooth discoid (resting) to spiny spheroid (activated), as seen in the images below. Morphology score measures this shape change, and ranges from 100 (all platelets are activated) to 400 (all platelets are resting). The swirl score measures the amount of shimmer in the platelets bag when swirled, which is affected by the shape of the platelets. The score ranges from 1 (no shimmer, indicating more platelet activation) to 4 (lots of shimmer, indicating fewer activated platelets). The extent of shape change (ESC) assay measures platelet responsiveness to adenosine diphosphate (ADP), an agonist that causes platelets to shift from discoid to spheroid. A lower ESC score indicates that many platelets are already spheroid (activated) and therefore do not change shape.
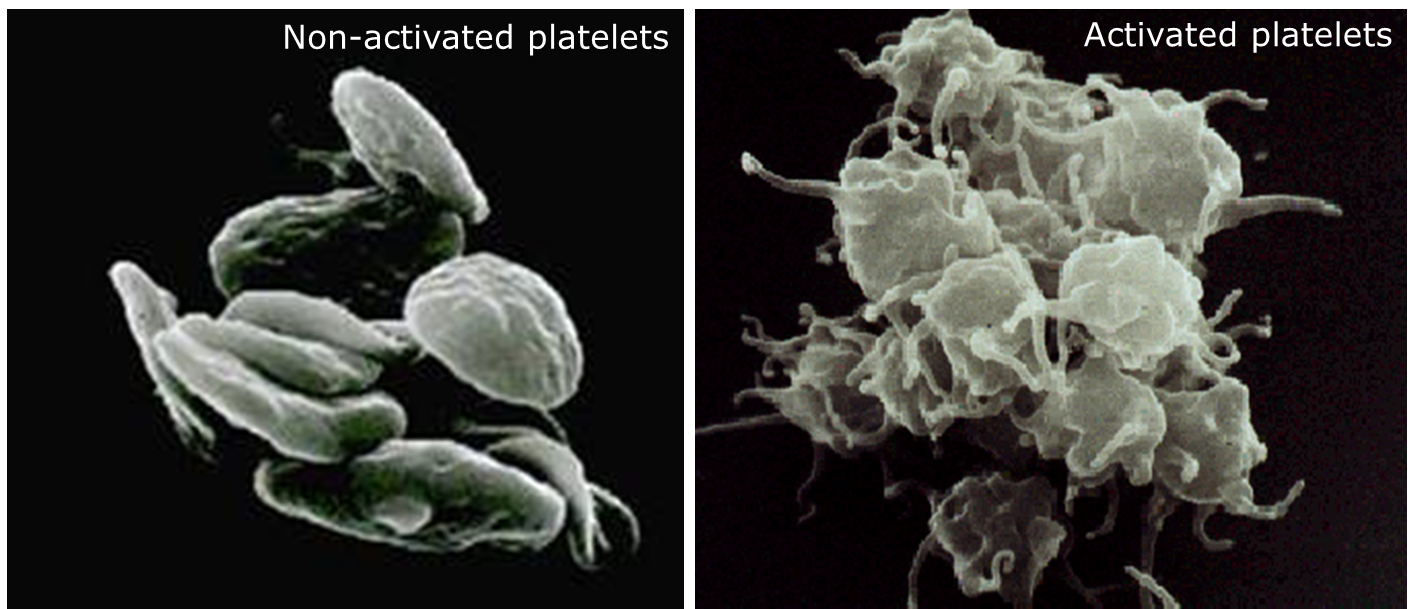
Table 2: Activation and responsiveness markers of buffy coat platelets at day five versus day seven of storage.
| Quality parameter | Day 5 | Day 7 | n | |
|---|---|---|---|---|
| Activation |
CD62P (% positive) |
33 ± 4 | 38 ± 4* | 26 |
| Morphology score (scale 100–400) | 243 ± 13 | 231 ± 14* | 4 | |
|
Swirl score (scale 1–4) |
2.5 ± 0.8 | 2.0 ± 0.9* | 13 | |
| Responsiveness |
Extent of shape change (%) |
34 ± 11 | 28 ± 7* | 13 |
|
Table shows mean values ± standard deviation. * p < 0.05, day five versus day seven, paired Student’s t test. |
Key observations
- Platelet activation increased, as shown by increased CD62P positivity and a decreased score for morphology and swirl.
- Platelet responsiveness decreased, as shown by a decrease in the extent of shape change in response to an agonist.
What do these changes mean?
All changes observed were small and consistent with known effects of storage. Platelet concentration in the bag remained constant between day five and day seven, maintaining platelet yield, a quality control requirement related to platelet concentration. The decrease in glucose and increase in lactate, along with the decreased pH, show that the platelets remain metabolically active between days five and seven of storage. The pH remained well within the range required by the Canadian Standards Association.1 Platelet activation increases throughout storage, as seen by a progressive decline in morphology score, swirl score and extent of shape change, along with increasing CD62P positivity.
For most variables, the difference between five and seven days of storage was smaller than the observed standard deviation. Consistent with the clinical demonstration that platelets stored for six or seven days show equivalent efficacy to platelets stored for five days or less,2 it is unlikely that these small changes would cause any functional differences in platelet behavior. Platelet products continue to fulfill all quality control requirements.
|
Quality control requirements for production of buffy coat platelets by Canadian Blood Services1, 3
Platelet yield: ≥240 × 109 in at least 75% of units tested pH: between 6.4 and 7.8 in at least 95% of units tested post-expiration Leukoreduction: <5 x 106 residual white blood cells in all units tested Sterility: no growth in all units tested post-expiration Volume: ± 10% of the labelled volume in all units |
How can this information inform clinical practice?
Although the factors studied here are standard markers of platelet quality used in research laboratories, they do not accurately predict patient outcomes after platelet transfusions.4, 5 Although the clinical trial of MacLennan et al.2 has shown non-inferiority for platelets stored for six or seven days compared to platelets stored for five days or less, this study was conducted in the UK, not in Canada. Since the platelet products prepared in the UK use identical methods to those produced by Canadian Blood Services, we expect similar clinical efficacy. The improved bacterial testing procedures that allow a seven-day shelf life are expected to increase the safety of platelet products, relieve inventory pressure, and reduce waste due to outdated products.

Related content
Platelet utilization and inventory management best practices
Buffy coat component production method
Acknowledgements
The authors acknowledge Rob Romans, BSc, ART, for his guidance on the content of this article and Drs. Kendra Hodgkinson, Geraldine Walsh, and Sophie Chargé from the Centre for Innovation’s Knowledge Mobilization and Education team for their editorial support.
References
1. Canadian Standards Association. Z902-15: Blood and blood components. CSA Group, 2015.
2. MacLennan S, Harding K, Llewelyn C, Choo L, Bakrania L, Massey E, Stanworth S, Pendry K, Williamson LM. A randomized noninferiority crossover trial of corrected count increments and bleeding in thrombocytopenic hematology patients receiving 2- to 5- versus 6- or 7-day-stored platelets. Transfusion 2015; 55: 1856-65.
3. Canadian Blood Services. Circular of information for the use of human blood components: pooled platelets LR CPD, apheresis platelets. Ottawa, ON, Canadian Blood Services, 2017.
4. Devine DV, Serrano K. The platelet storage lesion. Clin Lab Med 2010; 30: 475-87.
5. Kelly AM, Garner SF, Foukaneli T, Godec TR, Herbert N, Kahan BC, Deary A, Bakrania L, Llewelyn C, Ouwehand WH, Williamson LM, Cardigan RA. The effect of variation in donor platelet function on transfusion outcome: a semirandomized controlled trial. Blood 2017; 130: 214-20.