Solvent detergent (S/D) treated plasma (Octaplasma)
Authors: Shuoyan Ning, MD, FRCPC, DRCPSC; Isabelle Blais-Normandin, MD, FRCPC; Bryan Tordon, MD, FRCPC; Johnathan Mack, MD, MSc, FRCPC; and Kathryn Webert, MD, FRCPC
Primary target audiences: transfusion medicine physicians, non-transfusion medicine physicians, nurses, medical laboratory technologists in a hospital laboratory
NOTE: Please also see our FAQ on S/D treated plasma (Octaplasma).
Introduction
This publication provides information on Octaplasma—blood group specific, pathogen-inactivated human plasma for transfusion—based on its product monograph and evidence available in the literature. Octaplasma is manufactured by Octapharma using solvent detergent (S/D) treated pooled human plasma and is currently licensed for use in Canada. In 2011 Octaplasma was approved for use in select patient populations.1 Starting March 27, 2023, Octaplasma is available for routine use for all adult and pediatric patients requiring plasma transfusion. The goal of transitioning to a pathogen-reduced plasma supply as the main source for plasma transfusion is to provide an additional layer of protection for Canadian patients.
Octaplasma manufacturing and product description
Manufacturing
Octaplasma is a pooled human plasma formulation that is pathogen inactivated through solvent detergent (S/D) treatment. The S/D treatment involves thawing and pooling plasma donations from 630–1,520 individuals with the same ABO blood group (A, B, AB, or O).
The pathogen inactivation process used in S/D treatment differs from the process used at Canadian Blood Services to manufacture pathogen-reduced platelet components (for more information on pathogen-reduced platelets see Chapter 19 of the Clinical guide to transfusion). The S/D process used for plasma inactivates lipid-enveloped viruses, such as human immunodeficiency virus (HIV), hepatitis B, and hepatitis C. Other enveloped viruses (e.g., West Nile, Chikungunya, influenza strains) are also susceptible to S/D treatment.2
Stringent controls are applied by Octapharma to the selection and screening of donors for hepatitis B, hepatitis C, and HIV. The plasma pools are tested for hepatitis B surface antigen and anti-HIV 1/2 antibodies; nucleic acid testing is performed for hepatitis A, B, C, and E, HIV and parvovirus B19 prior to manufacturing. S/D treatment does not inactivate non-enveloped viruses, such as hepatitis A and parvovirus B19.3
Pooled plasma undergoes filtration steps after pooling to remove cells such as leukocytes and cell fragments. Plasma pools are treated with S/D reagents [1% tri(n-butyl) phosphate (TNBP) and 1% octoxynol] to inactivate lipid-enveloped viruses. The S/D reagents are removed, and the product is then applied through an affinity ligand resin column that selectively binds prion proteins. After sterile filtration, the final product is divided into individual units and stored at 18°C or colder (the same storage conditions as frozen plasma [untreated]). Octaplasma contains coagulation factors and other plasma proteins (albumin, immunoglobulins, other globulins, complement, protease inhibitors). Please refer to the product monograph for details.3
Packaging and storage
Octaplasma units are contained within sterile, plasticized polyvinyl chloride (PVC) bags over-wrapped with polyamide/polyethylene film. Each individual unit is packaged in a cardboard carton. Each unit contains 200 mL of S/D plasma. The product is stored frozen at ≤ -18°C or colder (the same storage conditions as frozen plasma [untreated]); when stored in these conditions, Octaplasma has a shelf life of 48 months. There are two transfusion ports, which are used to access the product. Blood group O, A, B, and AB plasma are available. Once thawed, the shelf life of Octaplasma is 5 days when stored at 2–8°C or 8 hours when stored at room temperature (20-25oC). Once thawed, Octaplasma must not be refrozen. Please refer to the product monograph for details.3
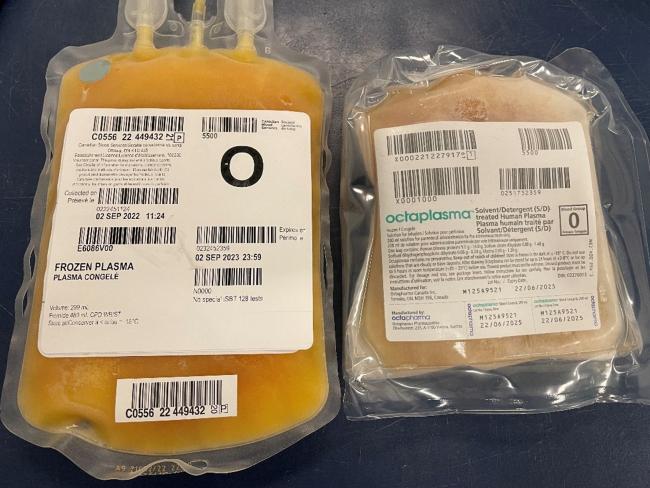
Figure 1. A frozen unit of Octaplasma (right) beside a unit of untreated frozen plasma (left). The Octaplasma bag is smaller and the plastic is thicker compared to frozen plasma (untreated). Each unit of Octaplasma has a volume of 200 mL. Please see the product monograph for thawing instructions.3
Dosing and product characteristics
Dosing is comparable to that of untreated frozen plasma (12–15 mL of Octaplasma/kg and 10–15 mL frozen plasma [untreated]/kg). For a 70 kg patient, 4–5 units of Octaplasma may be required to increase clotting factor levels by approximately 25%. Please refer to the product monograph for details.3
Octaplasma contains human plasma proteins, sodium citrate dihydrate, sodium dihydrogen-phosphate dihydrate, and glycine. The total human plasma protein concentration is between 45–70 mg/mL. In vitro studies have shown a mild reduction (10 –20%) of some coagulation factors and coagulation inhibitor activity in S/D plasma compared to untreated frozen plasma.2,4,5 All pathogen inactivation systems are known to lead to some reduction of coagulation and inhibitor activities6, with notable reductions in factors V and VIII across systems.7 However, it should be noted that all coagulation factor levels in Octaplasma remain within normal ranges expected for frozen plasma (untreated) and studies have not suggested clinical significance of minor clotting factor reductions; exceptions include protein S and alpha-2 antiplasmin (see the section on drawbacks and contraindications below), which are significantly reduced.
Individual coagulation factors in Octaplasma are > 0.5 IU/mL, even at day six of storage.3,8,9 All Octaplasma batches are routinely tested for factor V, factor VIII, and factor XI (specification > 0.5 IU/mL), as well as protein C (> 0.7 IU/mL), protein S (> 0.3 IU/mL), and alpha-2 antiplasmin (> 0.2 IU/mL).6
Table 1: Plasma protein levels of Octaplasma compared to fresh frozen plasma
| Parameters |
Octaplasma (n=12*) Mean (min-max) |
Reference ranges Fresh frozen plasma (FFP) |
|---|---|---|
| Total protein (mg/mL) | 55 (54–57) | 48–64 |
| Albumin (mg/mL) | 32 (30–34) | 28–41 |
| Fibrinogen (mg/mL) | 2.5 (2.4–2.6) | 1.45–3.85 |
| IgG (mg/mL) | 9.65 (9.15–10.10) | 6.60–14.50 |
| IgA (mg/mL) | 2.00 (1.80–2.05) | 0.75–4.20 |
| IgM (mg/mL) | 1.25 (1.20–1.30) | 0.40–3.10 |
| Factor V (IU/mL) | 0.78 (0.75–0.84) | 0.54–1.45 |
| Factor VII (IU/mL) | 1.08 (0.90–1.17) | 0.62–1.65 |
| Factor X (IU/mL) | 0.78 (0.75–0.80) | 0.68–1.48 |
| Factor XI (IU/mL) | 0.99 (0.91–1.04) | 0.42–1.44 |
| Protein C (IU/mL) | 0.85 (0.81–0.87) | 0.58–1.64 |
| Protein S (IU/mL) | 0.64 (0.55–0.71) | 0.56–1.68 |
| Plasmin inhibitor (IU/mL) | 0.23 (0.20–0.27) | 0.72–1.32 |
|
Source: Octaplasma product monograph.3 *12 consecutive batches of Octaplasma were investigated; mean (minimum–maximum) values are presented. |
||
Administration
The administration of Octaplasma must be ABO blood group compatible. Like frozen plasma, high dosages or infusion rates may induce hypervolemia, pulmonary edema, and/or cardiac failure. High infusion rates may also lead to citrate toxicity; due to this risk, the infusion rate should not exceed 1 mL Octaplasma/kg body weight/minute. The effect of citrate can be minimized by giving calcium gluconate using another line to administer. Please refer to the product monograph for details.3
Clinical use of S/D plasma
In 2011, S/D plasma became available for use in select patients requiring high volume or chronic plasma transfusions (congenital thrombotic thrombocytopenic purpura (TTP), plasmapheresis requiring plasma as replacement fluid, clotting factor deficiencies for which licensed concentrates are not readily available), and recurrent allergic reactions or an existing lung disorder with increased risk of transfusion related acute lung injury (TRALI). S/D plasma also became available for use in patients for whom group compatible plasma was not available, as well as for patients with a historical life-threatening reaction to plasma.1,10
Octaplasma has been available for use in select patient populations in Canada since 2011. On March 27, 2023, restrictions for ordering Octaplasma were removed and it is now available for routine use in all adult and pediatric patients requiring plasma transfusion. Octaplasma has been used widely in Europe for several decades, with some countries (e.g., Norway, Sweden, Finland, United Kingdom, The Netherlands) using Octaplasma as the primary plasma product for transfusion.
Hemostatic efficacy
There is limited evidence comparing S/D plasma to frozen plasma (untreated). Available evidence suggests that S/D plasma is comparable to frozen plasma (untreated) with respect to hemostatic efficacy. Clinical studies have shown that S/D plasma is effective in improving coagulation parameters and achieving clinical hemostasis in various patient populations.10-15 A 2016 systematic review found six randomized controlled trials (total of 561 patients) that compared efficacy and safety of S/D plasma to various other plasma formulations in common clinical scenarios (1 cardiac bypass, 3 liver transplantation, 1 coagulopathy, 1 prolonged prothrombin time). There were no clinically significant differences, but studies were small and underpowered.11 Among cardiac surgery patients with complex coagulopathies, coagulation parameters and clinical hemostasis were not different following transfusion of S/D plasma versus frozen plasma – with the exception of anticipated lower protein S and plasmin inhibitor activity.16 Of note, clotting factor levels in single donor plasma products and coagulation factor levels after frozen plasma infusions may be highly variable.5,17 A recently published cohort study comparing S/D and frozen plasma in a heterogenous patient population showed no differences in clinical outcomes (subsequent plasma requirements, transfusions of other blood components, length of hospital stay, in-hospital mortality) between the study groups.13 A pilot randomized trial comparing Octaplasma to standard plasma (VIPER-OCTA) for bleeding patients undergoing emergency thoracic aorta dissection surgery also reported favourable hemostatic outcomes.18
For factor V deficiency where no factor concentrates are available, in vitro studies have shown that S/D plasma is equivalent to untreated plasma in improving thromboelastrometry (ROTEM) indicators of hemostasis (EXTEM) and in increasing factor V.19 The use of S/D plasma resulted in a median factor V level of 25.5% (25.0–27.3%), comparable to the factor V increment when using untreated plasma 27.0% (20.0-29.5%). Small case series have also described the use of S/D plasma in treating other hereditary factor deficiencies with good clinical effect.20,21
Liver disease patients
There have been a small number of randomized trials and cohort studies examining the use of S/D plasma among liver cirrhosis patients and those undergoing liver transplantation. A randomized trial of 48 patients requiring plasma for invasive procedure or transplantation were randomized to receive either untreated frozen plasma or S/D plasma.22 Compared to frozen plasma, improved prothrombin time (PT) and no differences in activated partial thromboplastin time (aPTT) corrections were observed. No overt hemorrhagic complications were seen in both groups, and no patients required blood transfusion after the procedure. Two randomized controlled trials of patients undergoing liver transplantation (N = 293 total patients in one study23 and 63 total patients in the other24) comparing S/D plasma to frozen plasma showed no differences in correction of coagulopathy, intraoperative blood loss and overall clinical efficacy.23,24 Another study by Bindi et al., which used thromboelastography to guide transfusion among cirrhotic patients underlying transplant surgery, reported a significant reduction in the volume of plasma required in the S/D arm compared to the frozen plasma arm (i.e., 2,617 ± 1,297 mL for the frozen plasma arm versus 1,187 ± 560 mL for the S/D plasma arm, p <0.0001).24
Thrombotic thrombocytopenia purpura (TTP), hemolytic-uremic syndrome (HUS) with factor H deficiency
There are no published randomized data comparing S/D plasma to frozen plasma among patients with TTP or HUS. Biochemically, S/D plasma production process reduces high molecular weight von Willebrand factor multimers that trigger the shear stressed-induced platelets aggregation seen in TTP.25,26 A normal level of ADAMTS13 necessary for TTP therapy is retained in the S/D product. S/D plasma also retains factor H1122 – a regulatory protein in the alternative complement pathway that may be reduced in HUS.26
In lieu of randomized studies, case series and observational studies have provided safety data on the use of S/D plasma in these settings.25,27,28 In one retrospective cohort of 50 TTP episodes treated with plasma exchange using cryosupernatant or S/D plasma, no differences were noted in the median number of plasma exchanges to remission and volume of plasma used; less allergic and citrate reactions were also observed with the S/D product.27 The benefits of S/D plasma (outlined below in the section on benefits), including reduced allergic reaction and TRALI risks, are important considerations in this setting given the high volumes of plasma required.
Neonatal, pediatrics, and intrauterine transfusions
The safety and efficacy of S/D plasma among pediatric patients have been evaluated in clinical studies and from hemovigilance databases. Two recently published open label, post-market phase IV studies, reported on the use of S/D plasma among 50 critically ill patients (including 37 patients under age 2) with liver dysfunction and/or undergoing major surgery29, and 41 patients (age 2 and up) undergoing 102 plasma exchanges.30 S/D plasma was well tolerated overall, and not felt to be associated with any thrombotic or hyperfibrinolytic complications. A large observational study of 419 pediatric ICU patients (median age 1 year) among 101 pediatric ICUs in 21 countries also reported no safety concerns with S/D plasma. This study described post-transfusion international normalized ratio (INR) and ICU mortality, which were noted to be not different from patients transfused with untreated frozen plasma.31 Published retrospective cohorts have also reported on the short-term safety and efficacy of S/D plasma among neonates and pediatric patients.32-37 The youngest patients receiving S/D plasma were described in one study that included 136 extreme preterm infants of less than 28 weeks of age.33 Indications for S/D plasma transfusion varied between studies; clinical settings described were broad and included therapeutic plasma exchange, cardiac surgery, liver dysfunction, prevention of intracranial hemorrhage, and critical illness. European hemovigilance data from as early as the 1990s of S/D plasma have also been reassuring.2
Overall, short-term data suggest safety of S/D plasma among neonates and pediatric patients, but long-term data are currently limited. There are no published studies addressing the use of S/D plasma in intrauterine transfusions. Benefits and risks should be assessed and balanced before using S/D plasma in these settings. Please refer to the product monograph for details.3
Pregnant patients
There are limited studies describing the use of S/D plasma among pregnant patients. While there are no indications of harm, data are from small observational cohorts or case series.32,38-40 There are no harmful effects expected due to the TNBP and octoxynol in the product from animal studies. Please refer to the product monograph for details.3
Benefits
Reduces transfusion-transmitted infectious risks
The pathogen inactivation process reduces the risks of transfusion transmitted infections. S/D treatment damages lipid membranes and provides safety against lipid enveloped bacteria, protozoa, and viruses such as HIV, HBV, HCV, West Nile virus and Zika virus.11 While S/D treatment has no effect on non-enveloped viruses (such as hepatitis A and parvovirus B19), pooling of plasma reduces possible viral load through dilution and provides neutralizing antibodies.2,41 Nucleic acid testing is also performed for hepatitis A and parvovirus B19 for the plasma pools. The S/D treatment includes sterile filtration that depletes leukocytes and bacteria, as well as a column to remove prions – which reduces prion transmission risks.3,42-44
Reduces adverse transfusion reactions and TRALI risks
S/D plasma may lower risks of adverse transfusion reactions and TRALI compared to untreated plasma, possibly in part due to dilution of antigens, antibodies, and cytokines present in individual plasma units.11,45,46 Filtration steps used in S/D plasma production reduces bioactive particles, cell fragments and cytokines which may contribute to reactions.11
A 2019 clinical practice scientific review from the AABB identified 15 observational trials and 7 randomized controlled trials that evaluated the safety of S/D plasma. None of the studies identified an increased risk of adverse transfusion reactions, and allergic reaction rates were consistently lower with S/D plasma compared to untreated plasma.47 In France, a regional 10-year survey reported an allergic reaction rate of 4.86/10,000 S/D plasma transfusions compared to 7.14/10,000 untreated plasma transfusions.48 Hemovigilance data from UK, Norway, France, and Italy have shown similar low risks of reported adverse events11,49-51 with S/D plasma. From a TRALI risk perspective, the dilution of human leukocyte antigen (HLA) and human neutrophil antigen (HNA) antibodies52 reduces TRALI risks following S/D plasma transfusion compared to untreated plasma.47,50 There have however been rare cases of TRALI reported following transfusion with S/D plasma.53,54
Standardized clotting factor levels
Unlike single donor plasma, there is substantially improved standardization of factor levels in S/D plasma due to the pooling process.4,8,9,41,42,55-58 Untreated single donor plasma carry significant fluctuations in clotting factor content (factor variations between 50% to 200%). Studies have shown that clotting factor levels in and following untreated plasma infusions may be highly variable.17,5 All Octaplasma batches are routinely tested for Factor V, Factor VIII, and Factor XI (specification > 0.5 IU/mL), as well as protein C (> 0.7 IU/mL), protein S (> 0.3 IU/mL), and alpha-2 antiplasmin (> 0.2 IU/mL).3,6 Functionally, there are no differences in PT and aPTT corrections, changes in thrombin generation, or viscoelastic point-of-care resting results between these two products.16,17,24,55,59 Hemostatic efficacy of S/D plasma versus untreated plasma is considered equivalent and described above. The standardization of coagulation factor content in S/D plasma may be particularly beneficial to pediatric patients who receive plasma transfusions of lower volumes.
Drawbacks and contraindications
Reduced protein S and alpha2-antiplasmin content
There is significantly reduced protein S and alpha2-antiplasmin (a plasmin inhibitor) content in S/D plasma compared to untreated plasma. S/D treatment causes partial inactivation of protein S leading to reduced activity.4 In one study, the protein S level in S/D plasma was 0.41 U/mL on day 0 and deteriorated to 0.18 U/mL by day 6 of storage9 (range in frozen plasma 0.56-1.68 IU/mL); protein S requirements from the manufacturer for product release is > 0.3 IU/mL. For alpha2-antiplasmin, the activity is approximately 0.23 IU/mL (the range in frozen plasma is 0.72–1.32 IU/mL)3; the requirement for product release is > 0.2 IU/mL. As such, severe protein S deficiency is a contraindication for the use of S/D plasma.2,60 The clinical impact of reduced alpha2-antiplasmin levels is unclear. There are no reports of persistent bleeding in patients with congenital/acquired plasmin inhibitor deficiencies who received S/D plasma therapy.42 However, Octaplasma should not be used to correct hyperfibrinolysis caused by specific deficiencies in alpha2-antiplasmin. Please refer to the product monograph for details.3
Earlier formulations of S/D plasma produced in the U.S. (1998 –2002, produced by Vitex USA) were associated with bleeding and thromboembolic complications after liver transplantation and apheresis for TTP, leading to its withdrawal from the U.S. market in 2002. In comparison to European S/D plasma, the U.S. product had lower levels of alpha-2 antiplasmin and protein S, as well as other biochemical differences. These quality differences were attributed to differences in manufacturing and potentially contributed to complications observed with the U.S. product.47,61-64 The current formulation of Octaplasma has not been reported to be associated with an increased risk of bleeding or thrombosis.42 A cohort study examining hyperfibrinolysis and thrombosis complications in an S/D plasma transplant centre compared to untreated plasma centres showed no differences in these outcomes.65
Other considerations
There is paucity of long-term data among pediatric and neonates transfused with S/D plasma (literature reviewed above). There are no published studies of S/D plasma use for intrauterine transfusions. There are also limited studies describing the use of S/D plasma among pregnant patients, although published data suggests no harm.
S/D plasma is not considered IgA deficient. As per the product monograph, Octaplasma is contraindicated in patients with IgA deficiency with documented antibodies IgA as it may cause anaphylactic or anaphylactoid reactions. S/D plasma should also be avoided in patients who have hypersensitivities to the product or any ingredients in the formulation. Please refer to the product monograph for details.3
Additional resources
FAQ: Solvent detergent (S/D) treated plasma (Octaplasma)
- This FAQ was developed in collaboration with Octapharma to support hospitals in transitioning to pathogen-reduced plasma and reflects evidence available at time of publication.
Octaplasma (S/D plasma): One-page clinical summary
-
This one-page clinical summary can be printed by blood bank staff and provided with Octaplasma units when they are sent to the floor for transfusion.
(Slide deck) Octaplasma (Solvent detergent [S/D] plasma): Clinical overview
-
This slide deck may be downloaded for use in presentations. It provides health-care professionals with an overview of Octaplasma, including product characteristics, safety, indications and contraindications, and benefits of pathogen inactivation.
(Slide deck) Octaplasma (Solvent detergent [S/D] plasma): Clinical overview - Short version
-
This slide deck may be downloaded for use in presentations. It provides health-care professionals with a shorter version of the clinical overview, highlighting key points about Octaplasma.
April 4, 2023
(Slide deck) Octaplasma: Information for laboratory technologists
-
This slide deck may be downloaded for use in presentations. It provides laboratory technologists with an overview of information from the Octaplasma product monograph, including key points about labelling, packaging, storage and thawing.
NAC recommendations for the use of solvent-detergent plasma in Canada
- This document from the National Advisory Committee on Blood and Blood Products (NAC) provides additional clinical recommendations regarding the indications, dosing and use of S/D plasma in special populations.
Suggested citation
Ning S, Blais-Normandin I, Tordon B, Mack J, Webert K. Solvent detergent (S/D) treated plasma (Octaplasma) [Internet]. Ottawa: Canadian Blood Services; 2023 [cited YYYY MM DD]. Available from: https://profedu.blood.ca/en/solvent-detergent-sd-treated-plasma-octaplasma
References
- Canadian Agency for Drugs and Technologies in Health. Optimal therapy recommendation for the use of solvent/detergent-treated human plasma. CADTH Technol Overv 2, e2202 (2012).
- Hellstern, P. & Solheim, B.G. The Use of Solvent/Detergent Treatment in Pathogen Reduction of Plasma. Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie 38, 65-70 (2011).
- Octapharma Canada. Octaplasma Product Monograph, October 31, 2022. (Octapharma Canada, Toronto, ON, 2022).
- Beeck, H. & Hellstern, P. In Vitro Characterization of Solvent/Detergent-Treated Human Plasma and of Quarantine Fresh Frozen Plasma. Vox sanguinis 74, 219-223 (1998).
- Theusinger, O.M., Baulig, W., Seifert, B., et al. Relative concentrations of haemostatic factors and cytokines in solvent/detergent-treated and fresh-frozen plasma. British journal of anaesthesia 106, 505-511 (2011).
- Green, L., Bolton-Maggs, P., Beattie, C., et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. British Journal of Haematology 181, 54-67 (2018).
- Atreya, C., Glynn, S., Busch, M., et al. Proceedings of the Food and Drug Administration public workshop on pathogen reduction technologies for blood safety 2018 (Commentary, p. 3026). Transfusion 59, 3002-3025 (2019).
- Leebeek, F.W., Schipperus, M.R. & van Vliet, H.H. Coagulation factor levels in solvent/detergent-treated plasma. Transfusion 39, 1150-1151 (1999).
- Buchta, C., Felfernig, M., Höcker, P., et al. Stability of coagulation factors in thawed, solvent/detergent-treated plasma during storage at 4 degrees C for 6 days. Vox sanguinis 87, 182-186 (2004).
- National Advisory Committee on Blood and Blood Products. NAC Recommendations for the Use of Solvent Detergent Plasma in Canada. (National Advisory Committee on Blood and Blood Products, 2023).
- Marietta, M., Franchini, M., Bindi, M.L., et al. Is solvent/detergent plasma better than standard fresh-frozen plasma? A systematic review and an expert consensus document. Blood Transfus. 14, 277-286 (2016).
- Cicchetti, A., Berrino, A., Casini, M., et al. Health Technology Assessment of pathogen reduction technologies applied to plasma for clinical use. Blood transfusion = Trasfusione del sangue 14, 287-386 (2016).
- Racine-Brzostek, S.E., Canver, M.C., DeSimone, R.A., et al. Thawed solvent/detergent-treated plasma demonstrates comparable clinical efficacy to thawed plasma. Transfusion 60, 1940-1949 (2020).
- Spinella, P.C., Frazier, E., Pidcoke, H.F., et al. All plasma products are not created equal: Characterizing differences between plasma products. The journal of trauma and acute care surgery 78, S18-25 (2015).
- Canadian Agency for Drugs and Technologies in Health. Optimal Therapy Recommendation for the Use of Solvent/Detergent-Treated Human Plasma. in CADTH Optimal Use Report (Ottawa, ON, 2011).
- Haubelt, H., Blome, M., Kiessling, A.H., et al. Effects of solvent/detergent-treated plasma and fresh-frozen plasma on haemostasis and fibrinolysis in complex coagulopathy following open-heart surgery. Vox sanguinis 82, 9-14 (2002).
- Chowdary, P., Saayman, A.G., Paulus, U., et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. British journal of haematology 125, 69-73 (2004).
- Stensballe, J., Ulrich, A.G., Nilsson, J.C., et al. Resuscitation of Endotheliopathy and Bleeding in Thoracic Aortic Dissections: The VIPER-OCTA Randomized Clinical Pilot Trial. Anesthesia and analgesia 127, 920-927 (2018).
- Cushing, M.M., Asmis, L., Calabia, C., et al. Efficacy of solvent/detergent plasma after storage at 2-8 °C for 5 days in comparison to other plasma products to improve factor V levels in factor V deficient plasma. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis 55, 114-119 (2016).
- Inbal, A., Epstein, O., Blickstein, D., et al. Evaluation of solvent/detergent treated plasma in the management of patients with hereditary and acquired coagulation disorders. Blood Coagul Fibrinolysis 4, 599-604 (1993).
- Santagostino, E., Mancuso, M.E., Morfini, M., et al. Solvent/detergent plasma for prevention of bleeding in recessively inherited coagulation disorders: dosing, pharmacokinetics and clinical efficacy. Haematologica 91, 634-639 (2006).
- Williamson, L.M., Llewelyn, C.A., Fisher, N.C., et al. A randomized trial of solvent/detergent-treated and standard fresh-frozen plasma in the coagulopathy of liver disease and liver transplantation. Transfusion 39, 1227-1234 (1999).
- Bartelmaos, T., Chabanel, A., Léger, J., et al. Plasma transfusion in liver transplantation: a randomized, double-blind, multicenter clinical comparison of three virally secured plasmas. Transfusion 53, 1335-1345 (2013).
- Bindi, M.L., Miccoli, M., Marietta, M., et al. Solvent detergent vs. fresh frozen plasma in cirrhotic patients undergoing liver transplant surgery: a prospective randomized control study. Vox sanguinis 105, 137-143 (2013).
- Evans, G., Llewelyn, C., Luddington, R., et al. Solvent/detergent fresh frozen plasma as primary treatment of acute thrombotic thrombocytopenic purpura. Clinical and laboratory haematology 21, 119-123 (1999).
- Yarranton, H., Lawrie, A.S., Purdy, G., et al. Comparison of von Willebrand factor antigen, von Willebrand factor-cleaving protease and protein S in blood components used for treatment of thrombotic thrombocytopenic purpura. Transfusion medicine 14, 39-44 (2004).
- Scully, M., Longair, I., Flynn, M., et al. Cryosupernatant and solvent detergent fresh-frozen plasma (Octaplas) usage at a single centre in acute thrombotic thrombocytopenic purpura. Vox sanguinis 93, 154-158 (2007).
- de Wit, Y., Rethans, A., van Mierlo, G., et al. Plasma Exchange Therapy Using Solvent Detergent-Treated Plasma: An Observational Pilot Study on Complement, Neutrophil and Endothelial Cell Activation in a Case Series of Patients Suffering from Atypical Hemolytic Uremic Syndrome. Transfusion Medicine and Hemotherapy 49, 288-297 (2022).
- Spinella, P.C., Borasino, S. & Alten, J. Solvent/Detergent-Treated Plasma in the Management of Pediatric Patients Who Require Replacement of Multiple Coagulation Factors: An Open-Label, Multicenter, Post-marketing Study. Front Pediatr 8, 572 (2020).
- Josephson, C.D., Goldstein, S., Askenazi, D., et al. Safety and tolerability of solvent/detergent-treated plasma for pediatric patients requiring therapeutic plasma exchange: An open-label, multicenter, postmarketing study. Transfusion 62, 396-405 (2022).
- Camazine, M.N., Karam, O., Colvin, R., et al. Outcomes Related to the Use of Frozen Plasma or Pooled Solvent/Detergent-Treated Plasma in Critically Ill Children. Pediatr Crit Care Med 18, e215-e223 (2017).
- Chekrizova, V. & Murphy, W.G. Solvent-detergent plasma: use in neonatal patients, in adult and paediatric patients with liver disease and in obstetric and gynaecological emergencies. Transfusion medicine 16, 85-91 (2006).
- Al-Abdi, S., Dabelah, K., Mousa, T., et al. Selective prophylactic solvent-detergent plasma and cryoprecipitate transfusion to prevent intraventricular hemorrhage in extreme preterm infants: A case-historical control. J Neonatal Perinatal Med 11, 241-248 (2018).
- Witt, V., Pichler, H., Beiglboeck, E., et al. Changes in hemostasis caused by different replacement fluids and outcome in therapeutic plasma exchange in pediatric patients in a retrospective single center study. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis 56, 59-65 (2017).
- Kalsi, A.S., Al-Azzawi, O. & Gill, R. Comparison of the Coagulation Effect Achieved by OctaplasLG Versus Fresh Frozen Plasma in Pediatric Cardiac Surgical Patients. Clin Appl Thromb Hemost 24, 1327-1332 (2018).
- Liumbruno, G.M. & Franchini, M. Solvent/detergent plasma: pharmaceutical characteristics and clinical experience. J Thromb Thrombolysis 39, 118-128 (2015).
- Hall, S., Randall, K., Cole, R., et al. A paediatric tertiary referral centre experience of OctaplasLG (R). British journal of haematology 161, 66-66 (2013).
- Scully, M., Thomas, M., Underwood, M., et al. Thrombotic thrombocytopenic purpura and pregnancy: presentation, management, and subsequent pregnancy outcomes. Blood 124, 211-219 (2014).
- O'Connell, M.P., Eogan, M., Murphy, K.M., et al. Solvent-detergent plasma as replacement therapy in a pregnant patient with factor V deficiency. J Matern Fetal Neonatal Med 16, 69-70 (2004).
- Verghese, L., Tingi, E., Thachil, J., et al. Management of parturients with Factor XI deficiency-10year case series and review of literature. Eur J Obstet Gynecol Reprod Biol 215, 85-92 (2017).
- Hellstern, P. & Haubelt, H. Manufacture and composition of fresh frozen plasma and virus-inactivated therapeutic plasma preparations: correlation between composition and therapeutic efficacy. Thrombosis research 107 Suppl 1, S3-8 (2002).
- Liumbruno, G.M., Marano, G., Grazzini, G., et al. Solvent/detergent-treated plasma: a tale of 30 years of experience. Expert review of hematology 8, 367-374 (2015).
- Neisser-Svae, A., Bailey, A., Gregori, L., et al. Prion removal effect of a specific affinity ligand introduced into the manufacturing process of the pharmaceutical quality solvent/detergent (S/D)-treated plasma OctaplasLG. Vox sanguinis 97, 226-233 (2009).
- World Health Organization. WHO Guidelines on viral inactivation and removal procedures intended to assure the viral safety of human blood plasma products, Annex 4, TRS No 924. Vol. 2023 (World Health Organization, 2004).
- Saadah, N.H., van Hout, F.M.A., Schipperus, M.R., et al. Comparing transfusion reaction rates for various plasma types: a systematic review and meta-analysis/regression. Transfusion 57, 2104-2114 (2017).
- Henricks, L.M., Huisman, E.J., Lopriore, E., et al. Acute haemolytic transfusion reaction after transfusion of fresh frozen plasma in a neonate-Preventable by using solvent/detergent-treated pooled plasma? Transfusion medicine (2022).
- Cushing, M.M., Pagano, M.B., Jacobson, J., et al. Pathogen reduced plasma products: a clinical practice scientific review from the AABB. Transfusion 59, 2974-2988 (2019).
- Bost, V., Odent-Malaure, H., Chavarin, P., et al. A regional haemovigilance retrospective study of four types of therapeutic plasma in a ten-year survey period in France. Vox sanguinis 104, 337-341 (2013).
- Bolton-Maggs, P.H.B., New, H.V. & Tinegate, H. Use of and reactions to fresh frozen plasma in the UK. ISBT Science Series 11, 133-139 (2016).
- Flesland, O. A comparison of complication rates based on published haemovigilance data. Intensive care medicine 33 Suppl 1, S17-21 (2007).
- Steinsvåg, C.T., Espinosa, A. & Flesland, Ø. Eight years with haemovigilance in Norway. What have we learnt? Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis 49, 548-552 (2013).
- Sachs, U.J., Kauschat, D. & Bein, G. White blood cell-reactive antibodies are undetectable in solvent/detergent plasma. Transfusion 45, 1628-1631 (2005).
- Klanderman, R.B., Bulle, E.B., Heijnen, J.W.M., et al. Reported transfusion-related acute lung injury associated with solvent/detergent plasma - A case series. Transfusion 62, 594-599 (2022).
- Klanderman, R.B., van Mourik, N., Eggermont, D., et al. Incidence of transfusion-related acute lung injury temporally associated with solvent/detergent plasma use in the ICU: A retrospective before and after implementation study. Transfusion 62, 1752-1762 (2022).
- Heger, A., Neisser-Svae, A., Trawnicek, L., et al. Thrombin generation potential and clot-forming capacity of thawed fresh-frozen plasma, plasma frozen within 24 h and solvent/detergent-treated plasma (octaplasLG(®) ), during 5-day storage at 1-6°C. Vox sanguinis (2018).
- Irsch, J., Pinkoski, L., Corash, L., et al. INTERCEPT plasma: comparability with conventional fresh-frozen plasma based on coagulation function--an in vitro analysis. Vox sanguinis 98, 47-55 (2010).
- Mintz, P.D., Bass, N.M., Petz, L.D., et al. Photochemically treated fresh frozen plasma for transfusion of patients with acquired coagulopathy of liver disease. Blood 107, 3753-3760 (2006).
- Singh, Y., Sawyer, L.S., Pinkoski, L.S., et al. Photochemical treatment of plasma with amotosalen and long-wavelength ultraviolet light inactivates pathogens while retaining coagulation function. Transfusion 46, 1168-1177 (2006).
- Lerner, R.G., Nelson, J., Sorcia, E., et al. Evaluation of solvent/detergent-treated plasma in patients with a prolonged prothrombin time. Vox sanguinis 79, 161-167 (2000).
- Hellstern, P. Solvent/detergent-treated plasma: composition, efficacy, and safety. Current opinion in hematology 11, 346-350 (2004).
- Neisser-Svae, A. & Heger, A. Two solvent/detergent-treated plasma products with a different biochemical profile. ISBT Science Series 11, 94-101 (2016).
- Yarranton, H., Cohen, H., Pavord, S.R., et al. Venous thromboembolism associated with the management of acute thrombotic thrombocytopenic purpura. British journal of haematology 121, 778-785 (2003).
- Salge-Bartels, U., Breitner-Ruddock, S., Hunfeld, A., et al. Are quality differences responsible for different adverse reactions reported for SD-plasma from USA and Europe? Transfusion medicine 16, 266-275 (2006).
- Flamholz, R., Jeon, H.R., Baron, J.M., et al. Study of three patients with thrombotic thrombocytopenic purpura exchanged with solvent/detergent-treated plasma: is its decreased protein S activity clinically related to their development of deep venous thromboses? Journal of clinical apheresis 15, 169-172 (2000).
- Haugaa, H., Taraldsrud, E., Nyrerød, H.C., et al. Low incidence of hyperfibrinolysis and thromboembolism in 195 primary liver transplantations transfused with solvent/detergent-treated plasma. Clin Med Res 12, 27-32 (2014).