Chapter 14
Therapeutic apheresis
Background
Apheresis, derived from the Greek “ἀφαίρεσις / aphairesis” meaning “to carry away” is the process whereby whole blood is removed from an individual and separated into its components. A specified component is retained and the remainder is returned to the individual. Therapeutic apheresis is used to treat patients with a variety of disorders and has become an increasingly common treatment modality. The indications, rationale, and techniques for therapeutic apheresis procedures, as well as the care of the apheresis patient, will be reviewed in this chapter.
Therapeutic apheresis procedures
Therapeutic apheresis procedures include therapeutic plasma exchange (TPE), cytapheresis (including red cell exchange), and extracorporeal photopheresis.
Therapeutic plasma exchange
TPE is used to treat a variety of conditions caused by harmful substances (usually antibodies) which accumulate in a patient’s plasma. Treatment with TPE is based on these assumptions:
- The disease is caused by a circulating pathogenic substance.
- The pathogenic substance can be efficiently removed from the blood.
- Removal or reduction in the amount of the pathogenic substance will lead to improvement or resolution of disease manifestations.
TPE may be used as the primary treatment (e.g., immune-mediated thrombotic thrombocytopenic purpura [TTP]) or as an adjunct to other therapies (e.g., anti-glomerular basement membrane disease).
The efficacy of TPE depends on numerous variables including the volume of plasma removed relative to the patient's total plasma volume, volume of distribution of the substance, the plasma protein binding affinity of the substance to be removed, and the number and frequency of procedures. TPE is effective at removing predominantly intravascular proteins and those that equilibrate rapidly between the intravascular and extravascular spaces. Finally, the rate at which the pathogenic substance is synthesized is important. The best therapeutic results of TPE is when the substance is synthesized slowly.1
The American Society for Apheresis (ASFA) guidelines describe the role of therapeutic apheresis using the following categorization. The clinical disorders for which therapeutic apheresis is considered an acceptable, standard first-line therapy or a valuable adjunct therapy are Category I indications (Table 1). Category II indications are disorders for which apheresis is accepted as a second-line therapy, either alone or in combination with other modalities. Indications for which an optimum role of apheresis has not been established and those for which apheresis has been found to be ineffective or harmful are designated as Categories III and IV, respectively.2
Table 1: Category I indications for therapeutic apheresis (ASFA 2019 guidelines2)
|
Therapeutic plasma exchange
|
|
Cytapheresis
|
Cytapheresis
Cytapheresis can be used to deplete a pathologic cellular blood component or for collection of specific cells. This includes red cell exchange (depletion of red blood cells), leukopheresis (depletion of white blood cells) and thrombocytapheresis or plateletpheresis (depletion of platelets). Cytapheresis allows collection of a specific subset of cells for autologous or allogeneic therapies (e.g., mononuclear cell [MNC] collection for stem cell transplantation or lymphocytes for the purpose of chimeric antigen receptor T-cell [CAR-T] therapy).
Note that cytapheresis is also used to collect and manufacture allogeneic apheresis platelet products including HLA- and HPA-selected products.
Red cell exchange
Red cell exchange, also known as erythrocytapheresis involves removal of a patient's pathologic red blood cells and replacement with donor red blood cells. This can be performed manually or in an automated fashion using a cell separator. The most common indication is management of complications related to sickle cell disease. For description of a manual red cell exchange and for sample calculations, please refer to Table 1 in Swerdlow3. Usually 1.5 red cell volumes are exchanged. For automated red cell exchange, the device will calculate the volume of donor red blood cells required on the basis of patient’s sex-at-birth, height, weight, initial and final desired hematocrits, fluid balance, and desired fraction of cells remaining (i.e., the percentage of the patient’s red blood cells remaining in the circulation post-procedure). The red cell volume to be exchanged depends on the desired fraction of cells remaining for the procedure, which in turn depends on the underlying condition. Patients with sickle cell disease should ideally receive red blood cell units that are selected for RhD/C/E and Kell as well as for any other antigens based upon any allo-antibodies they may have developed, informed by their red cell genotyping and serological studies.
Indications for red cell exchange include: sickle cell disease with acute stroke (Category I, see Table 1), acute chest syndrome (Category II), primary or secondary stroke prophylaxis (Category I, see Table 1), and severe babesiosis (Category II).
Leukocytapheresis
Leukocytapheresis may be indicated in some patients with hyperleukocytosis and symptomatic leukostasis (Category II). Leukostasis is the result of microvascular obstruction by leukocytes which can lead to endothelial injury and hemorrhage or thrombosis of end organs, mostly commonly the brain and lungs. Signs and symptoms of leukostasis can arise in patients with acute leukemia and circulating white blood cell counts exceeding 50 x 109/L, but more commonly >100 x 109/L. Leukocytapheresis rapidly lowers the white blood cell count and can potentially reverse symptoms of leukostasis, particularly if there is a delay in starting cytoreductive chemotherapy. Usually 1.5–2.0 blood volumes are processed, and patients may require fluid replacement to maintain hemodynamic stability. Prompt initiation of chemotherapy is paramount to prevent hyperleukocytosis from recurring. Prophylactic leukocytapheresis is not shown to be beneficial and not recommended by the ASFA guidelines.2
Thrombocytapheresis
Thrombocytapheresis may be indicated as a second-line therapy (Category II) in patients with thrombocytosis due to a myeloproliferative neoplasm causing acute and severe complications such as thrombosis or hemorrhage. Thrombocytapheresis can rapidly lower the platelet count and improve symptoms while waiting for cytoreductive therapy to take effect. The procedure can be repeated as necessary. Usually 1.5–2.0 blood volumes are processed; an intra-procedural complete blood count (CBC) may be of value to confirm that the desired target reduction in platelet count (usually below 400 x 109/L) has been achieved.
Extracorporeal photopheresis
Finally, extracorporeal photopheresis is an immunomodulatory apheresis intervention. During photopheresis, peripheral blood MNC are collected, exposed extracorporeally to 8-methoxypsoralen followed by UVA photoactivation, then re-infused into the patient. In most cases, this occurs within a single, closed-circuit device. Onset of effect is gradual, with median time to effect from weeks to months. ECP is most commonly used to treat cutaneous T-cell lymphoma (CTCL), Sézary syndrome, and recalcitrant acute and chronic graft-versus-host disease (GvHD) following allogeneic stem cell transplantation.
Centrifugation versus membrane filtration
The two main techniques for the separation of blood components during apheresis are centrifugation and membrane filtration.
Centrifugation
Centrifugal apheresis can be used to remove cellular components and is very efficient, achieving plasma extraction of nearly 80%. One of the benefits of centrifugal apheresis is that it requires lower blood flow rates and therefore can be performed using either peripheral or central venous access. Citrate (e.g., ACD-A) is the most commonly used anticoagulant. Centrifugation can be intermittent or continuous.
-
Intermittent flow centrifugation involves the processing of small volumes of blood in cycles (a cycle consists of blood withdrawal, processing, and reinfusion). The advantage of using an intermittent flow instrument includes use of single site venous access; however, the procedure time is longer and larger fluctuations in extracorporeal blood volume and hemodynamics occur as compared with continuous flow devices.
-
Continuous flow centrifugation involves the simultaneous removal, processing, and re-infusion of blood components. Continuous flow devices have the advantage of faster procedures and more hemodynamic stability but require two sites of vascular access or a central apheresis line with two lumens.
Membrane filtration
Membrane filtration devices allow for the selective removal of high molecular weight proteins by altering pore sizes of membranes and can be used as an alternative to centrifugal apheresis. These devices (e.g., dialysis machines) are usually more readily accessible than centrifugal separators. Some disadvantages of membrane filtration devices include the need to use heparin as an anticoagulant and the requirement of higher blood flow rates necessitating central vascular access.1 Additionally, these devices are less efficient than centrifugal separators as they have much lower plasma extraction (about 30%) which increases procedure times. Finally, membrane filtration devices are not suitable for cytapheresis.
Selective adsorption columns
Apheresis devices can also be fitted with a column that selectively removes substances of interest, including lipoproteins, specific cells like activated monocytes or granulocytes, and immunoglobulins (e.g., IgG, isohemagglutinins). Adsorption techniques take advantage of presenting ligands that are specific for the pathogenic substances of interest or even pathologic substance specific electrostatic interactions, allowing other important and non-pathogenic components to be returned to the patient. This can be highly advantageous in instances where more efficient removal of pathologic substances can be achieved without the requirement for replacement with human plasma products. Due to limited availability and cost, adsorption columns are not widely available for clinical use in Canada. Select sites to utilize ABO-specific columns to facilitate ABO-incompatible organ transplants (see sections 2.7.1 and 2.7.2 in the 2019 ASFA guidelines2) or lipoprotein columns for treatment of familial hypercholesterolemia.
Care of the therapeutic apheresis patient
Therapeutic apheresis is an invasive procedure with the potential for significant clinical consequences. Apheresis centres should ideally have a quality management system including standard operating policies and procedures, qualified apheresis practitioners, appropriately licensed machines with regular preventative maintenance, and a mechanism to report and investigate adverse events.
Any patient requiring apheresis warrants a thorough medical history, physical examination, and relevant laboratory investigations. The focus of the medical history should be the indication for apheresis to ensure the request is appropriate and the patient’s ability to tolerate the procedure. The patient’s diagnosis, symptoms, comorbidities, current medications, and concurrent treatments should be explored to identify potential complications and interactions so they can be mitigated. The physical examination should include vital signs, height and weight, venous access assessment, and volume status. The indication for apheresis and patient’s diagnosis should guide the remainder of the physical exam. Prior to the first apheresis procedure, laboratory testing should include: a complete blood count; creatinine; electrolytes including calcium, magnesium, phosphate; albumin and liver transaminases. Additional laboratory studies such as antibody and serologic testing, and biochemical disease markers may be required depending on the indication for apheresis. These should be drawn prior to the first treatment to ensure accurate baseline results.
Apheresis patients are often immunosuppressed and exposed to large numbers of blood components. An assessment for immunity against hepatitis B and offering vaccination if non-immune is prudent. All apheresis patients should undergo a review of primary infection prophylaxis candidacy and strategies. Hence, it is beneficial to work in a multidisciplinary team that includes pharmacists and, when appropriate, infectious disease specialists.
Informed consent for apheresis requires that the patient or substitute decision-maker be fully aware of the indication(s), alternatives, risks, and potential benefits of treatment. Furthermore, an additional, separate consent to receive blood and blood products is required.4 Once the apheresis has commenced, the treatment plan should be reviewed and adjusted regularly based on clinical progress and patient tolerance.
Indications
Therapeutic apheresis is used to treat a wide range of disease processes and disorders. The ASFA publishes evidence-based guidelines (last updated in 2019),2 which succinctly but comprehensively outline indications, practical considerations, and suggested frequency and duration of treatment. The 2019 ASFA guidelines review the published apheresis literature for >80 medical conditions and >150 clinical indications.
Vascular access
Vascular access is required to perform apheresis procedures.5 For centrifugal apheresis, access may employ peripheral or central veins, or a combination of the two. For adults, flow rates of 60–120 ml/minute are required to ensure an exchange can be completed in a reasonable time period (ideally <3 hours). The type of vascular access chosen must be able to withstand lumen collapse from significant draw pressure. Thus, peripherally inserted central catheters, Hickman or Broviac central venous catheters (CVC) or small peripheral IV catheters (>20 gauge) are not suitable for apheresis.
Suitable options for vascular access include peripheral veins (16–18 gauge preferred), double-lumen CVC designed for apheresis or hemodialysis, subcutaneous implantable access device (e.g., Vortex port-a-cath), or arteriovenous fistula or graft. Access through peripheral veins is the modality of choice in most cases because it is associated with fewer infectious, thrombotic, and insertion-related complications (e.g., discomfort, scarring, bleeding or pneumothorax) as compared to central lines. Increasingly, point-of-care, ultrasound-guided peripheral IV insertion is being used by apheresis nurses. Application of this technique in some centres has resulted in the majority of apheresis procedures being done with peripheral access.6 However, in patients requiring chronic apheresis or daily procedures over a prolonged period of time, for example for acute TTP patients, central access may still be required.
Technical problems with apheresis catheters such as leakage or inadequate flow rates can often be resolved by repositioning or rewiring the catheter. Infusion of local fibrinolytic agents may also be required.
Technical notes
Typically, 1.0–1.5 plasma volumes are exchanged with each TPE procedure, which translates into the removal of approximately 65%–80% of intravascular plasma constituents. Calculation of plasma volume should incorporate the patient’s sex-at-birth, height, weight and hematocrit. Numerous formulae have been published (e.g., online calculator based on Nadler’s method7); alternatively, plasma volume can be estimated using the calculation in Figure 1 below. Certain patient populations may require special considerations when calculating plasma volume such as: individuals who are pregnant, obese, who have had amputations or are on sex-hormone supplementation (e.g., testosterone), and in pediatrics.
The urgency, frequency and duration of apheresis interventions are guided by the underlying disease process, indication for apheresis, and the patient’s response to therapy. Emergent indications for TPE include TTP, other acute thrombotic microangiopathies, and stroke or end-organ failure in the context of sickle cell disease. Most other indications for apheresis can be initiated within 24–72 hours depending on the patient’s clinical status and underlying disease process. The frequency and duration of treatment is dependent on the nature of the target substance to be removed (e.g., IgM or IgG antibody) and the desired clinical endpoint (recovery of symptoms, bridge to definitive therapy, etc.). Furthermore, the apheresis physician must balance the need for aggressive removal of the pathogenic substance versus allowing the substance to re-equilibrate into the intravascular space. A typical plasma exchange course of four to six treatments over 10–14 days can reduce IgG by 70–85%.8 In contrast, IgM is approximately 80% intravascular and thus usually 1–2 exchanges provide sufficient relief of acute hyperviscosity symptoms. For most indications, there is variability in practice with respect to specific frequency and duration of the apheresis course. For example, for TTP, there is agreement that plasma exchange should be performed daily until the platelet count has exceeded 150 × 109 /L; however, practice variability remains and there is some uncertainty about whether to taper TPE thereafter.9,10
A TPE order should include the following information:
-
Indication
-
Number of plasma volumes (PV) to be exchanged
-
Replacement fluid to be used
-
Vascular access type & site
-
Circuit anticoagulant ratio (also known as AC ratio)
-
Calcium replacement orders
-
Final desired fluid balance
-
Frequency and estimated total number of treatments including clinical endpoint.
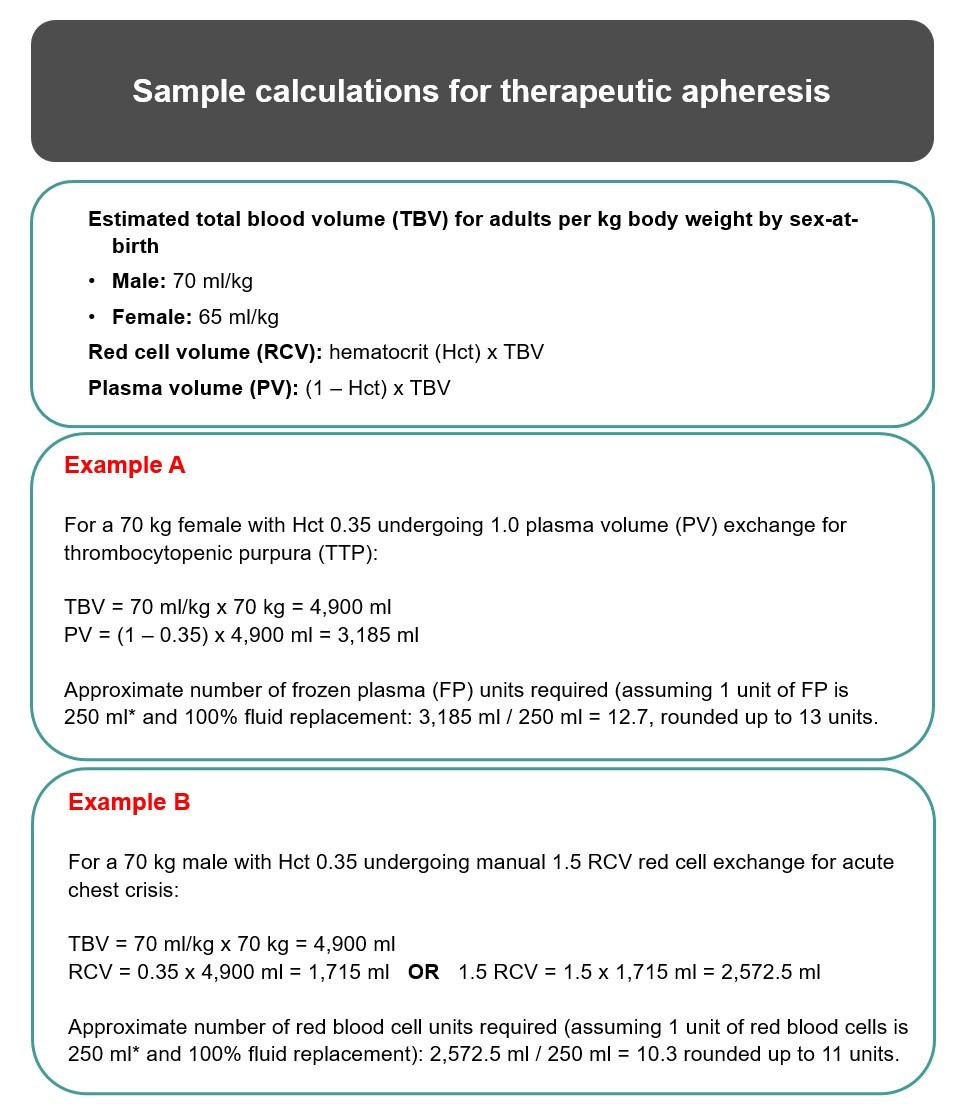
*Approximation only. Please refer to Canadian Blood Services’ Circular of Information and consult with hospital transfusion services for accurate estimates of the volume of frozen plasma7 and red blood cell units.10
Replacement fluids for apheresis
For TPE, the most commonly used replacement fluid is 5% albumin. Plasma replacement is used less frequently, and only with the intent to both remove a pathologic substance and replace deficient or dysfunctional components (e.g., ADAMTS13, clotting factors). Plasma replacement options include: frozen plasma CPD, apheresis fresh frozen plasma (sodium citrate), apheresis frozen plasma (ACD-A), cryosupernatant plasma CPD, and solvent detergent plasma (SDP)11 (see Chapter 2 for a description of Canadian Blood Services’ plasma components). Some centres may also use normal saline in combination with albumin or plasma, although this is becoming increasingly uncommon. An ideal replacement solution exerts colloid osmotic pressure equivalent to plasma to prevent hypotension, edema, and other adverse reactions.1
The advantages and disadvantages of each replacement fluid are summarized below:
-
Albumin (5% human albumin solution) can be used in a one-to-one replacement ratio and is a good long-term volume expander with a half-life of 17 days. Albumin is readily available, cost-effective, and generally well tolerated. It is solvent detergent treated and ultrafiltered to prevent transmission of infectious pathogens. Complications may include fever, bradykinin-induced hypotension in the setting of angiotensin-converting enzyme (ACE) inhibitors, hypokalemia, and dilutional coagulopathy.
-
Plasma replaces both coagulation factors and immunoglobulins. It is associated with a higher risk of transmitting pathogens relative to albumin, though this risk remains very low in Canada due to vigorous screening, collection, and processing (see Chapter 6 for information on Canadian Blood Services’ donor selection, donor testing and pathogen reduction). Other disadvantages include the need for ABO matching and increased processing time to thaw frozen products. Plasma also carries a higher risk of febrile and allergic transfusion reactions, as well as transfusion-related acute lung injury (TRALI). Finally, citrate contained in plasma in addition to the circuit anticoagulation can lead to increased citrate toxicity and hypocalcemia.
- Solvent detergent plasma (S/D plasma) is pooled plasma that undergoes specialized processing to inactivate potentially harmful pathogens, cells and antibodies. These processes result in a decreased risk of allergic reactions and immunologic transfusion reactions such as TRALI (for more information see our FAQ on S/D plasma at Canadian Blood Services). It is primarily used as a replacement fluid for patients with thrombotic microangiopathies, and/or when there is a history of severe allergic transfusion reaction(s) or pre-existing lung conditions.
- Cryosupernatant plasma (CSP), in jurisdictions where it may be available, can be used as a replacement fluid for patients with TTP. CSP is devoid of the largest von Willebrand factor multimers thought to be pathogenic in TTP, and thus theoretically offers an advantage over frozen plasma for these patients.12-14 However, in prospective studies comparing CSP and fresh frozen plasma (FFP in the management of TTP, CSP has not shown a clear survival advantage.15 Of note, CSP is no longer available through Canadian Blood Services; please see Canadian Blood Services’ customer letter (# 2025-02) for more information.
In general, TPE with plasma is reserved for treatment of patients with TTP to replace deficient ADAMTS13. However, plasma may also be indicated for patients who are actively bleeding, coagulopathic, or pre- or peri-operative to replete clotting factors. In these instances, plasma may be used alone or in combination with 5% albumin solution.
For red cell exchange, the replacement fluid is donor red blood cells. Antigen matching is particularly imperative for sickle cell patients to minimize alloimmunization. Extended matching (beyond Rh and K) is ideal; however, obtaining sufficient numbers of matched units can be challenging and lead to treatment delays. Thus, extended matching is usually reserved for patients with a history of multiple alloantibodies, hyperhemolysis, or delayed hemolytic transfusion reactions. Some institutions also use plasma reduced RBC units in this population. In addition, for patients receiving chronic automated exchanges, depletion-exchange may provide an opportunity to achieve the same clinical outcome with reduced product requirements.
Adverse events associated with apheresis
The rate of adverse events (AEs) during apheresis is 4–5%, with the risk being slightly higher for the first procedure.16 Adverse events may be related to complications from numerous factors including vascular access, replacement fluid, underlying disease process and type of procedure performed. The World Apheresis Association assessed more than 50,000 procedures and found the most common severe AEs were hypotension and urticaria. No deaths were reported.16 Mild and moderate AEs were reported with vascular access problems such as hematoma or repeated puncture attempts and tingling sensation, respectively. Unpublished data from the Canadian Apheresis Group (CAG) showed an overall decline in the incidence of adverse events from 2013– 2020.
Citrate toxicity is the most common AE with apheresis procedures. The mechanism is related to citrate-induced binding of ionized calcium and subsequent hypocalcemia. This can be compounded by the binding of serum calcium to 5% albumin when albumin is used as replacement. Other patients who are highly susceptible include those with severe renal or liver impairment or those receiving higher citrate load due to concomitant transfusion of red cells or frozen plasma. Hypocalcemia manifests clinically as perioral or peripheral paresthesia, flushing, lightheadedness, nausea or vomiting, carpopedal spasm, and, in severe cases, cardiac arrhythmias and seizures. In patients with renal insufficiency, infusion of citrate can also lead to metabolic alkalosis. It is standard to infuse calcium chloride or calcium gluconate concurrently with citrate during apheresis to prevent citrate toxicity. Citrate-to-replacement product ratio can also be titrated to symptom improvement.
TPE with albumin replacement depletes coagulation factors, including fibrinogen; however, this is not typically associated with clinically significant bleeding (5% risk)17, and clotting factors are restored to baseline levels 24–72 hours following TPE. Daily exchanges with albumin may also lead to hypogammaglobulinemia and increased infection risk. Immunoglobulins generally return to the pre-treatment levels in about 3–4 weeks if not on concomitant immunosuppression. Additionally, TPE, regardless of replacement fluid, may remove medications and pharmacologic metabolites. Drugs that are highly protein-bound and have small volumes of distribution are especially susceptible to removal by TPE, as are pharmaceutical antibodies (e.g., rituximab, eculizumab, IVIG). These should be given following completion of the apheresis course or avoided if possible.
Risks of red cell exchange are the same as those of massive red blood cell transfusion: febrile or allergic reactions, alloimmunization, citrate toxicity, TRALI, and transmission of infectious pathogens (see Chapter 10 on adverse reactions).
Other complications of apheresis include hypovolemia or vasovagal reactions; cellular losses (iron deficiency anemia and thrombocytopenia); and increased bleeding risk related to coagulation abnormalities with the use of non-plasma replacement fluids. Adverse events and the frequency of these events are listed in Table 2. Severe adverse events (primary reason in 168 procedures) resulting in interruption of apheresis are listed in Table 3.16
The treatment of AEs is guided by the type and severity of the reaction and preventive measures are essential. Mild allergic symptoms can be managed with peri- or intra-procedure antihistamines or corticosteroids. Patients with thrombotic microangiopathies and allergic symptoms are often switched to SDP. Hypotension can be prevented by withholding blood pressure medications (especially ACE inhibitors) prior to TPE, and by correcting anemia and hypovolemia with RBC transfusion and intravenous fluids, respectively. Cautiously, targeting a positive fluid balance post-procedure may be effective for patients who are chronically hypotensive despite these other strategies. To ensure the optimal care of the apheresis patient, close communication between the primary treating physician and the apheresis physician is encouraged, as is a supportive, multidisciplinary team environment.
Table 2: Adverse events associated with therapeutic plasma exchange (TPE). Adapted from Kaplan (1999).12
| Category | Symptom | Incidence (%) |
|---|---|---|
| Common events | <10% | |
| Hypocalcemia | Parasthesias | 1.5–9.0 |
| Hypovolemia | Hypotension Muscle cramps Headaches |
0.4–4.2 |
| Anaphylactoid | Urticaria Rigors |
0.7–12.0 1.1–8.8 |
| Rare events | ~1.5% | |
| Cardiac | Myocardial ischemia or infarction or shock Arrhythmia |
0.1–1.5 0.1–0.7 |
| Pulmonary Hematologic |
Respiratory arrest/pulmonary edema Pulmonary embolism Thrombosis/hemorrhage |
0.2–0.3 0.1 0.02–0.7 |
| Infectious | Hepatitis Other infection |
0.7 0.3 |
| Neurologic | Seizures Cerebrovascular ischemia |
0.03–0.4 0.03–0.1 |
| Miscellaneous | Hyperthermia | 0.7–1.0 |
Table 3: Severe adverse events (primary reason in 168 procedures) resulting in interruption of apheresis given as specified AEs/10,000 procedures.16
| Symptom, reason | AEs |
|---|---|
| Hypotension, syncope | 11 |
| Urticaria | 6 |
| Fever, chills | 3 |
| Nausea, vomit | 2 |
| Access problem | 2 |
| Flush | 2 |
| Tingling, stitching | 2 |
| Arrhythmia | 2 |
| Bronchospasm | 1 |
| Quincke edema | 1 |
| Technical problem | 0.8 |
| Abdominal pain | 0.8 |
| Back pain | 0.8 |
| Epilepsy | 0.6 |
| Hypertension | 0.4 |
| Spasm | 0.4 |
| Asystolia | 0.2 |
| TRALI chest pain | 0.2 |
| Anaphylaxis | 0.2 |
| Gastro intestinal bleeding | 0.2 |
| Wrong plasma | 0.2 |
| Adverse event to drug | 0.2 |
| Chest pain | 0.2 |
| Anxiety + hyperventilation | 0.2 |
Summary
Therapeutic apheresis, an umbrella term that encompasses TPE, red cell exchange and other cytapheresis procedures, and extracorporeal photopheresis, is used to treat patients with a variety of medical conditions. In general, the procedures are safe, effective, and well-tolerated but patients must be counselled and closely monitored for potential complications. Apheresis procedures should be performed by specially trained nurse operators and patients should be followed by physicians familiar with apheresis medicine. The American Society for Apheresis (ASFA) publishes evidence-based guidelines on the therapeutic use of apheresis in clinical practice. The field of apheresis continues to expand and evolve to meet the needs of a diverse patient population.
Continuing professional development credits
Fellows and health-care professionals who participate in the Canadian Royal College's Maintenance of Certification (MOC) program can claim the reading of the Clinical Guide to Transfusion as a continuing professional development (CPD) activity under Section 2: Individual learning. Learners can claim 0.5 credits per hour of reading to a maximum of 30 credits per year.
Medical laboratory technologists who participate in the Canadian Society for Medical Laboratory Science’s Professional Enhancement Program (PEP) can claim the reading of the Clinical Guide to Transfusion as a non-verified activity.
Acknowledgements
The authors acknowledge Katerina Pavenski MD, FRCPC; Megan Buchholz, RN; and Aditi Khandelwal, MDCM, FRCPC, DRCPC, for their review of this chapter. Katerina Pavenski and Nadine Shehata authored the previous version of this chapter.
Suggested citation
Oliver M and Patriquin C. Therapeutic apheresis. In: Khandelwal A, Abe T, editors. Clinical Guide to Transfusion [Internet]. Ottawa: Canadian Blood Services, 2022 [cited YYYY MM DD]. Chapter 14. Available from: https://professionaleducation.blood.ca
If you have questions about the Clinical Guide to Transfusion or suggestions for improvement, please contact us through the Feedback form.
References
- Williams, M.E. & Balogun, R.A. Principles of separation: indications and therapeutic targets for plasma exchange. Clinical journal of the American Society of Nephrology : CJASN 9, 181-190 (2014).
- Padmanabhan, A., Connelly-Smith, L., Aqui, N., et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice – Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. Journal of clinical apheresis 34, 171-354 (2019).
- Swerdlow, P.S. Red cell exchange in sickle cell disease. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program, 48-53 (2006).
- Canadian Standards Association Group. CAN/CSA-Z902:20 - Blood and blood components, (CSA, Canada, 2020).
- Golestaneh, L. & Mokrzycki, M.H. Vascular access in therapeutic apheresis: update 2013. Journal of clinical apheresis 28, 64-72 (2013).
- Barth, D., Nemec, R.M., Cho, D.D., et al. The practical integration of a hybrid model of ultrasound-guided peripheral venous access in a large apheresis center. Journal of clinical apheresis 35, 328-334 (2020).
- EasyCalculation.com. Blood volume calculator. Vol. 2022.
- Holcomb, J.B., Jenkins, D., Rhee, P., et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. The Journal of trauma 62, 307-310 (2007).
- Patriquin, C.J., Clark, W.F., Pavenski, K., et al. How we treat thrombotic thrombocytopenic purpura: Results of a Canadian TTP practice survey. Journal of clinical apheresis 32, 246-256 (2017).
- Patriquin, C.J. & Pavenski, K. Plasma exchange in TTP: to taper or not to taper. Transfusion 60, 1647-1648 (2020).
- Canadian Blood Services. Circular of Information for the Use of Human Blood Components: Plasma Components. (Canadian Blood Services, 2022).
- Kaplan, A.A. Therapeutic apheresis for renal disorders. Therapeutic apheresis : official journal of the International Society for Apheresis and the Japanese Society for Apheresis 3, 25-30 (1999).
- Hori, Y., Hayakawa, M., Isonishi, A., et al. ADAMTS13 unbound to larger von Willebrand factor multimers in cryosupernatant: implications for selection of plasma preparations for thrombotic thrombocytopenic purpura treatment. Transfusion 53, 3192-3202 (2013).
- Rock, G. The management of thrombotic thrombocytopenic purpura in 2005. Semin Thromb Hemost 31, 709-716 (2005).
- Zeigler, Z.R., Shadduck, R.K., Gryn, J.F., et al. Cryoprecipitate poor plasma does not improve early response in primary adult thrombotic thrombocytopenic purpura (TTP). Journal of clinical apheresis 16, 19-22 (2001).
- Mörtzell Henriksson, M., Newman, E., Witt, V., et al. Adverse events in apheresis: An update of the WAA registry data. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis 54, 2-15 (2016).
- Soares Ferreira Júnior, A., Boyle, S.H., Kuchibhatla, M., et al. Bleeding outcomes of inpatients receiving therapeutic plasma exchange: A propensity-matched analysis of the National Inpatient Sample. Transfusion 62, 386-395 (2022).