ABO subgroups
Authors: Youness Elkhalidy, MD, and Gwen Clarke, MD, FRCPC
Publication date: October 2020
Primary target audiences: Medical laboratory technologists (MLT) in a hospital laboratory, transfusion medicine physicians
The purpose of this document is to clarify the relevance of ABO subgroups for hospital blood banks selecting a red blood cell unit for transfusion and importance of working with Canadian Blood Services to investigate potential donors with an ABO subgroup.
Key points
- The ABO blood group system includes subgroups with weak expression of A or B antigen on red cells.
- Quantitative and/or qualitative differences in ABO subgroups can result in irregularities or discrepancies observed during ABO typing.
- During ABO confirmation testing of a red blood cell unit by the hospital blood bank, a weak reaction may be an indication of an ABO subgroup.
- A weak reaction observed by the hospital blood bank should be confirmed with Canadian Blood Services. If a subgroup has previously been identified through Canadian Blood Services’ routine donor testing, the red blood cell unit is safe to use as labelled; if not previously identified, the unit should not be issued.
What are ABO subgroups?
The A and B blood group antigens are produced by enzymes that modify glycoproteins found on the surface of red blood cells. The precursor glycoprotein to both the A and B antigens is known as the H antigen. As with other blood group systems, the ABO system has variant phenotypes with a genetic basis. The term “subgroup” refers to phenotypes with variations in the structure or number of the A and B antigens, related to variations in the enzymes that produce them. A and B subgroups can result in either of the following:
- Reduced numbers of structurally typical A and B antigens.
- Production of slightly altered A and B antigens, which may or may not be reduced in number.
Overall, A subgroups are much more common than B subgroups. Several A subgroups have been described, although the A2 subgroup is the most commonly recognized. Some individuals with an A subgroup may form antibodies to the typical A antigen (also known as A1 antigen) because of either quantitative (i.e., they produce so little A antigen) or qualitative differences (i.e., A subgroup phenotypes are distinguished from the A1 phenotype by glycolipid antigen expression).1, 2
How do ABO subgroups affect blood donor testing?
ABO subgroups can result in irregularities or discrepancies observed during ABO typing of a red blood cell unit. For instance, a subgroup that leads to reduced expression of A antigen, such the A2 subgroup, may result in a weaker forward reaction during typing. Other variants can result in mixed field reactions, which is the case with the A3 and B3 subgroups. A minority of subgroups may result in discrepancies between forward and reverse typing with an unexpected reverse typing, such as an anti-A1 in a group A2 individual.
However, it should be noted that many factors other than ABO subgroups can also affect serologic investigations, leading to difficulties in interpretation and ABO discrepancies. For instance, certain disease states can result in altered expression of ABO antigens or reduced production of naturally occurring ABO antibodies. Medication, passive antibodies, or cold-reacting antibodies may also interfere with testing. It is important to consider all factors that could possibly interfere with serological test results in order to ensure accurate ABO typing of red blood cell units and safe transfusions.
Some of the various ABO subgroups and their effects on forward and reverse grouping are shown in Table 1. Although not included in the table, subgroups can also occur in AB phenotypes. Blood group AB individuals with an associated A or B subgroup will frequently show ABO discrepancies in forward and reverse grouping. A relatively common example is an A2B person with an anti-A1, where the forward group shows a reaction with both anti-A and anti-B, but the reverse group has an unexpected reaction with A1 reagent cells.
Table 1: Various ABO subgroups and the ways they affect red blood cell typing (wk = weak, mf = mixed field)3
|
Phenotype |
Forward typing | Reverse typing | |||||||
| Anti-A |
Anti-B |
Anti-AB | Anti-A1 | Anti-H† | A1 cells‡ | A2 cells | B-cells | ||
| A1 (typical) | 4+ | - | 4+ | 4+ | - | - | - | 4+ | |
| A2 | 3-4+* | - | 4+ | - | 2-3+ | +/- | - | 4+ | |
| A3 | 2+/mf | - | mf/wk | - | 3-4+ | +/- | - | 4+ | |
| Ax | wk/- | - | 1-2+ | - | 4+ | 1+ | - | 4+ | |
| Aend | mf/- | - | mf | - | 4+ | +/- | - | 4+ | |
| Ael | - | - | - | - | 4+ | +/- | - | 4+ | |
| B (typical) | - | 4+ | 4+ | - | 4+ | 4+ | 4+ | 0 | |
| B3 | - | 2+/mf | wk | - | 4+ | 4+ | 4+ | 0 | |
| Bx | - | wk | 2+ | - | 4+ | 4+ | 4+ | 0 | |
| Bel | - | - | - | - | 4+ | 4+ | 4+ | 0 | |
|
† Anti-H (a lectin that agglutinates the ABO precursor molecule) can be used to assess the relative “strength” of an A subgroup. The normal A1-enzyme is very efficient at converting H to A1 such that very little, undetectable amounts of H are left behind. For A subgroups, the more detectable unaltered H-precursor is left (i.e., stronger anti-H reaction in forward typing), the weaker the A subgroup; more H antigen indicated relative inefficiency in converting H to the A subgroup antigen, or in other words, a “weak” phenotype. This is not applicable for B-subgroups as the normal B-enzyme is not as efficient at converting the H precursors to the B antigen and usually leaves significant amounts of unaltered H-antigens. As such, the presence of anti-H is not useful in distinguishing a typical B type from a B subgroup. ‡ For routine blood bank testing A1 cells are the stereotypical cells used in most ABO typing. The presence of agglutination to A1 cells in the reverse typing indicated that an A subgroup individual is able to make antibodies to the normal A1 antigen. * Although the A2 subgroup is considered a weak A subgroup, it usually does not affect the forward typing. Instead it is most commonly identified via the presence of an unexpected anti-A or anti-A1. |
|||||||||
As part of routine donor typing, Canadian Blood Services investigates all weak or discrepant reactions to resolve the discrepancies and ensure accurate reporting of the ABO blood group. Often subgroup investigations follow identification of weak reactions seen during forward typing. A weak reaction is defined as <2+ agglutination either by solid phase or manual methods. As part of routine work up, weak reactions are enhanced by altering cellular concentration, prolonging the incubation time and/or decreasing the incubation temperature. These are the “wk” reactions seen in Table 1. Anti-A1 specific lectins are also utilized to identify the presence or absence of the A1 antigen. Cells lacking the A1 antigen are presumed to contain an A subgroup. Mixed field reactions may also help to identify a A3 or B3 subgroup (see Table 1). In some cases, further testing at a reference lab is required, including adsorption and elution techniques, flow cytometric immunophenotyping to enumerate the antigens, or sequencing of the ABO genes.
Donors identified with blood groups corresponding to A2, A3 or B3 are labeled as either A or B units depending on the subtype, serologic studies and presence or absence of an anti-A1 antibody. If a red blood cell unit with an ABO subgroup is assigned an A/B typing, it is safe to use as labelled (e.g., like any other unit of that A/B typing). If weak or discrepant reactions cannot be resolved, or if an ABO subgroup incompatible with transfusion is identified, the donor will be deferred.
How can hospital blood banks determine if a donor has been evaluated for an ABO subgroup?
As required by the Canadian Standards Association (CSA Z902-20)4, hospital blood banks must perform forward typing confirmation of red blood cell units received by Canadian Blood Services in order to allow for release of units via electronic cross match. If a weak reaction (less than 2+) is identified during ABO confirmation testing, the unit may have an ABO subgroup.
In these cases, it is important to contact Canadian Blood Services distribution to ascertain whether a subgroup was identified and investigated by Canadian Blood Services donor testing during routine typing procedures. A Canadian Blood Services technical specialist can view donor history and confirm whether a subgroup was identified.
If Canadian Blood Services’ records confirm that a subgroup was identified, then the weak reaction seen during a hospital’s confirmation testing is an expected result of the ABO subgroup; the unit is then safe to use according to the ABO type on the end label (e.g., an A3 unit can be transfused to a group A individual). However, if no history is found, a code will be added to the donor’s file which will cause a manual workup to be done on the next donation to identify a possible ABO subgroup or other interference. In cases when a weak reaction is unexpectedly observed during confirmation testing (i.e., the subgroup has not been previously identified by Canadian Blood Services), the unit should not be issued. A hospital customer feedback form and return of the unit to Canadian Blood Services may be required and discussion with a hospital transfusion medicine physician is recommended.
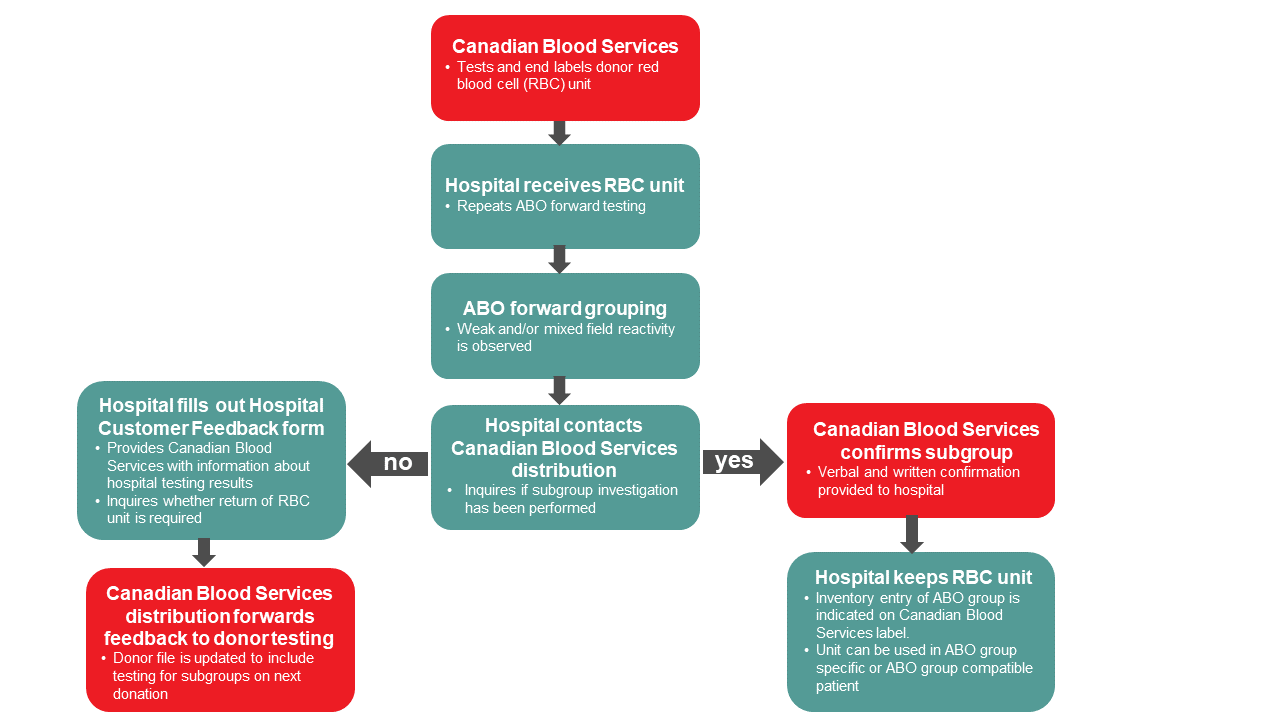
Figure 1. Algorithm for hospital blood banks for follow up of potential ABO subgroup donor units
Additional resources
For an introduction to immunohematology and the foundations of blood bank compatibility testing, visit LearnSerology.ca, an online educational resource developed by transfusion medicine specialists in Canada. The curriculum consists of six modules and includes an interactive module for completing an antibody investigation panel.
References
- Svensson L, Rydberg L, De Mattos LC, Henry SM. Blood Group A1 and A2 Revisited: An Immunochemical Analysis. Vox Sang 2009; 96: 56-61. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1423-0410.2008.01112.x.
- Daniels G. Human Blood Groups, 3rd Edition. Published by Wiley-Blackwell, 2013.
- Thakral B, Saluja K, Bajpai M, Sharma RR, Marwaha N. Importance of Weak Abo Subgroups. Lab Med 2005; 36: 32-4. https://doi.org/10.1309/X59TAAYPEPCNBLUJ.
- CSA Group. Can/Csa-Z902:20 - Blood and Blood Components. Published in Canada by CSA, 2020.