Whole blood donors with antibodies
Author: Matthew Yan, MD, FRCPC
Publication date: January 2019
Primary target audience: Medical laboratory technologist (MLT) in the hospital laboratory
Background
A very small percentage of red blood cell (RBC) units (< 0.5%) are manufactured from blood donors with red cell antibodies. These donors may have antibodies directed against common non-ABO antigens (e.g. Rh or Kell) detected during routine screening or may represent a rare blood donor with the corresponding antibody. Since 2010, Canadian Blood Services has standardized the issuing of RBC units with antibodies to all hospitals it serves (Figure 1). However, given the small percentage of RBC units with antibodies, some centers may not have experience in the handling and transfusion of these units. In general, these RBC units are considered safe to transfuse for all adult populations, regardless of the patient’s RBC phenotype, and should be avoided in pediatric patients.
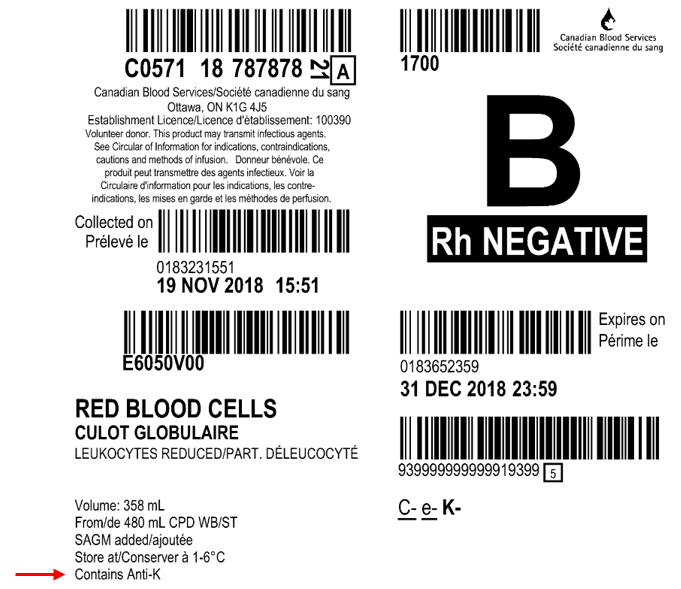
Figure 1. Example of a product label for a RBC unit from a donor with known antibodies. The red arrow indicates the label location for RBC antibody information. For common antibodies, the specificity will be provided (e.g. “Contains Anti-K). For antibodies against rare antigens, “Other-AB” will be printed on the label. If the specificity has not been determined, “UNID-AB” will be printed on the label.
Safety of units
RBC units with donor RBC antibodies are considered safe for transfusion in the adult population. These units are not typically transfused into the pediatric population as a precaution given the small blood volume of the patients.1 The safety of units is largely due to the small volume of residual plasma present in the RBC unit, which is further diluted by additives (e.g. SAGM) and by the large plasma volume of the transfusion recipient. RBC units manufactured by Canadian Blood Services typically contain less than 29 mL of residual plasma volume.2 This plasma volume is diluted by approximately 110 mL of SAGM additive.2
To evaluate the effect of additive dilution on passive antibodies, Nobiletti et al.3 examined the supernatant of 169 Adsol RBC units from donors with known antibodies. They found that 46% of the units had no detectable antibody. In 54% of the units with detectable antibodies, further five-fold dilution of the supernatant rendered the antibody undetectable. Similarly, Hill et al.4 examined donor testing samples and RBC unit segment samples from 39 donors with known antibodies. There was a median two-tube decrease in the titer of the antibody in the segment sample compared to the donor sample. The antibodies were undetectable in the segment sample in 28% of the cases.
Another feature that contributes to the safety of these RBC units is the low titer of the antibodies in the residual plasma of the units. Hill et al.4 reported a median titer of 1 in the RBC unit segments from their study of 39 donors. This is in contrast to anti-A and anti-B titers from group O RBC segments, which were found to be much higher with a median titer of 32. Despite having a higher titer, group O RBCs are commonly transfused to non-O recipients without much concern for hemolysis. This may be in part due to the presence of ABH substances present on tissue and plasma negating the effect of the antibodies, in addition to the mitigating factors already mentioned.
Theoretical calculation
Consider the worst-case scenario of a unit from a donor with a high-titer antibody transfused into a small female recipient (i.e. blood volume is smaller than average patient’s blood volume). Assuming the donor has an antibody with a titer of 1024 that is present in the residual plasma at an amount of 29 mL. Dilution with 110 mL of SAGM results at least in a two-tube decrease to a titer of 256 (based on the study by Hill et al.4). This volume of 139 mL (residual plasma + additive) is then transfused into a 150-cm 45-kg female. The blood volume of the recipient is approximately 2900 mL. Assuming this is an inappropriate transfusion, and the recipient is not anemic and therefore has a hematocrit of 44%, the plasma volume in the recipient is approximately 1600 mL. This would theoretically result in a further 11-fold dilution of the 139 mL from the RBC unit that contained antibodies. In all likelihood, this dilution would render any antibody insignificant.
Examples in practice
Since Canadian Blood Services standardized the distribution of RBC units from donors with antibodies in 2010, there have not been any reported hemolytic reactions from these units. The few rare case reports of hemolysis from the published literature involved whole blood units, which contain a significant amount of plasma, and differ from current production methods used in Canada. In these reports, inter-donor incompatibility occurred when an antigen-negative recipient received an antigen-positive unit followed by a unit containing the corresponding antibody.5-8
In the only published report evaluating the practice of transfusing antibody positive RBC units, Combs et al.9 assessed the clinical impact of 259 RBC units from donors with a total of 312 antibodies. Approximately 90% of the units had clinically significant antibodies. Of the 99 recipients with post-transfusion samples, only 10 had detectable passive antibodies, but no evidence of a hemolytic reaction. The authors concluded the practice of transfusing antibody positive RBC units to adult recipients was safe with minimal impacts on the hospitals’ workload. This practice allows the continued inclusion of blood donors with antibodies in Canadian Blood Services’ donor pool and thus contributes to the continued sufficiency of the blood supply for Canadians.
Additional resources
For an introduction to immunohematology and the foundations of blood bank compatibility testing, visit LearnSerology.ca, an online educational resource developed by transfusion medicine specialists in Canada. The curriculum consists of six modules and includes an interactive module for completing an antibody investigation panel.
References
- Garratty G. Problems associated with passively transfused blood group alloantibodies. Am J Clin Pathol 1998; 109: 769-777.
- Canadian Blood Services. Circular of Information for the Use of Human Blood Components: Red Blood Cells, Leukocytes Reduced (Lr). Published on www.blood.ca. Accessed on December 3, 2018.
- Nobiletti J, Badon S, Cable R, et al. Unexpected red cell antibodies are not detected in 46% of additive red cells from antibody positive donors. Transfusion 1998; 38 (Suppl):87S.
- Hill E, Bryant B. Comparison of antibody titers in donor specimens and associated AS-1 leukoreduced donor units. Transfusion 2014; 54: 1580-1584.
- Abbott D, Hussain S. Intravascular coagulation due to interdonor incompatibility. Can Med Assoc J. 1970; 103: 752-753.
- Franciosi RA, Awer E, Santana M. Interdonor incompatibility resulting in anuria. Transfusion. 1967; 7: 297-298.
- West NC, Jenkins JA, Johnston BR, et al. Interdonor incompatibility due to anti-Kell antibody undetectable by automated antibody screening. Vox Sang. 1986; 50: 174-176.
- Zettner A, Bove J. Hemolytic transfusion reaction due to interdonor incompatibility. Transfusion 1963; 3: 48-51.
- Combs MR, Bennett DH, Telen MJ. Large-scale use of red blood cell units containing alloantibodies. Immunohematology 2000; 16: 120-123.