Testing and management of fetal and neonatal alloimmune thrombocytopenia
Authors: Danielle Meunier, MD and Gwen Clarke, MD, FRCPC
Date of publication: May 2020
Primary target audiences: Medical laboratory technologists (MLT) in a hospital laboratory, transfusion medicine physicians
Key points
- Fetal and neonatal alloimmune thrombocytopenia (FNAIT) is an immune-mediated cause of thrombocytopenia in neonates due to maternal anti-human platelet antigen (HPA) antibodies, most commonly anti-HPA-1a.
- Intracranial hemorrhage (ICH) is a serious complication of FNAIT and is estimated to affect 1 in 10,000 live births.
- Diagnostic testing for FNAIT includes three main steps:
- Maternal anti-HPA antibody screening and identification
- HPA genotyping of mother, father and/or neonate
- Confirmation of antibody specificity and reactivity with the monoclonal antibody immobilization of platelet antigens (MAIPA) assay
- HPA-matched platelets should be given to a neonate with FNAIT if immediately available, otherwise unmatched units (random pooled platelets) should be provided until matched platelets can be obtained.
Neonatal thrombocytopenia
Thrombocytopenia in a neonate is defined as a platelet count of less than 150 x 109/l and is considered severe if the platelet count falls below 50 x 109/l.1 Neonatal thrombocytopenia may be caused by decreased platelet production (often congenital), or more commonly, it is due to increased destruction/consumption of platelets caused by, for example, sepsis, medications, necrotizing enterocolitis, disseminated intravascular coagulation (DIC), placental insufficiency, and asphyxia. Increased platelet consumption can also be immune-mediated via FNAIT, or rarely by maternal immune/idiopathic thrombocytopenic purpura (ITP).2
What is FNAIT?
Fetal and neonatal alloimmune thrombocytopenia is an uncommon but potentially serious complication of pregnancy caused by immune-mediated destruction of fetal platelets by maternal alloantibodies. It can occur any time there is an incompatibility between the fetal and maternal platelets from a paternally inherited antigen. FNAIT has an estimated incidence of 1 in 1,000 to 1 in 1,500 live births, however the true incidence is likely higher as it is often not considered in cases of miscarriage or intrauterine fetal demise.3-5 It is thought to be the most common cause of severe thrombocytopenia in otherwise healthy term neonates.6
The clinical presentation of FNAIT is highly variable and may include asymptomatic thrombocytopenia; mild bleeding in the form of petechiae, hematomas, small visceral bleeds, transient hematuria, or bloody stools; or severe bleeding, including major organ bleeds and ICH.1, 7, 8 Intracranial hemorrhage occurs in 10–25% of diagnosed FNAIT cases, with many cases being detected in utero in the late second and third trimesters.9 It is a feared complication of FNAIT as it may result in lifelong neurologic deficits or fetal/neonatal death.5
HPA and FNAIT
The human platelet antigen (HPA) epitopes are expressed on five key glycoproteins on the platelet membrane surface. Thirty-seven HPAs are currently described, 22 of which are generally considered clinically relevant (Table 1). The glycoproteins, such as GPIIIa, GPIb, and GPIa and GPIIa, are involved in essential platelet functions, including adhesion, aggregation and platelet plug formation. Antibodies against HPA-1a, an epitope of GPIIIa, are the most common cause of FNAIT and are implicated in approximately 80% of FNAIT in Caucasians.7, 10 This is followed by anti-HPA-5b, -HPA-15b and -HPA-3a, which are implicated in 10–15%, 4% and 1–2% of cases respectively. Other HPA antigens have been implicated at rates of less than 1%. In Asian populations, anti-HPA-5b is the most common cause of FNAIT, followed by anti-HPA-4b.11
The incidence of FNAIT due to anti-HPA-1a is illustrated in Figure 1. Although HPA-1a-negative pregnancies are common, ICH from FNAIT is much less common.
Table 1: Selected HPA antigens and their corresponding glycoprotein and cluster of differentiation (CD) number. Adapted from Metcalfe, 2004.6
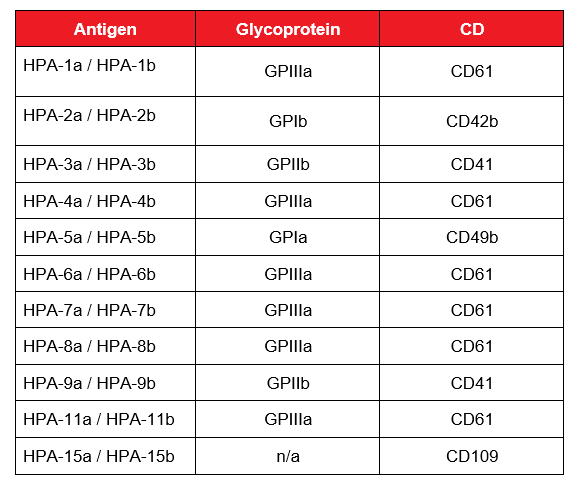
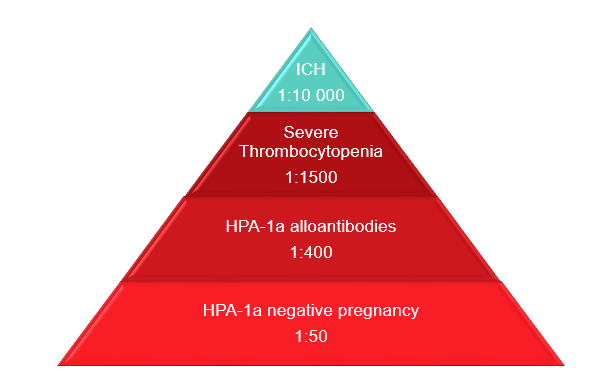
Figure 1: Epidemiology of FNAIT due to anti-HPA-1a. Adapted from de Vos et al. 2019.5 Licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/) Colours changed from the original.
Pathophysiology of FNAIT
The mechanism of FNAIT is similar to that of hemolytic disease of the fetus and newborn (HDFN). Maternal exposure to an incompatible, paternally inherited HPA on fetal platelets triggers alloimmunization and formation of anti-HPA antibodies in the mother. Since these are IgG antibodies, they readily cross the placenta and bind fetal platelets, which are then cleared from circulation via phagocytosis. Some anti-HPA antibodies, including anti-HPA-1a may also cause decreased platelet production due to megakaryocyte suppression.12 This leads to thrombocytopenia in the fetus and increased risk of bleeding.
The degree of thrombocytopenia does not always correlate to the severity of bleeding events; many neonates with FNAIT are severely thrombocytopenic and do not bleed, whereas others are only moderately thrombocytopenic but have catastrophic ICH. Although not fully understood, we know that HPAs are not exclusive to platelets but are expressed on other tissues as well, including endothelium.1 Animal models and ex-vivo studies suggest that the maternal anti-HPA antibodies cause direct damage to the endothelium.13-16 Anti-HPA-1a in particular, has also been shown to inhibit angiogenesis, leading to decreased vessel density in the brain and retinas and increased risk of bleeding at those sites.14
Unlike HDFN, alloimmunization of the mother to HPA tends to occur early on in pregnancy and FNAIT frequently affects the first pregnancy of an alloimmunized woman.5 Although fetal platelet antigens are expressed as early as 16 weeks gestation, HPA-1a is expressed even earlier on other fetal cells, such as placental chorionic villi.7, 17 The amount of HPA-1a on first trimester placenta is similar to that at term, suggesting a potential source for very early maternal alloimmunization.17 There is also an association of anti-HPA formation with HLA-DRB3*01:01.18 It is rare for a woman without this HLA type to form anti-HPA antibodies, and if she does, the resulting thrombocytopenia in her infant is generally mild.3, 19 Thus, this test is useful for its negative predictive value and can be used to help rule out the possibility of severe FNAIT.
HPA testing
Canadian Blood Services’ National Platelet Immunology Reference Laboratory (NPIRL) in Winnipeg, Manitoba performs HPA and HLA (human leukocyte antigen) typing and antibody investigations testing for many jurisdictions across Canada. These investigations are used clinically to evaluate FNAIT as well as platelet refractoriness, post-transfusion purpura and platelet function disorders.
Platelet donor testing
NPIRL performs HPA and HLA typing for all platelet donors before they are entered into the national platelet registry. Serological typing of HPAs is technically difficult to do; therefore, HPA genotyping is used to predict the phenotype. NPIRL uses the BioArray HPA BeadChip assay (Immucor, Warren, NJ, USA), which uses targeted probes to determine the genotype for the 22 different HPA antigens listed in Table 1. Canadian Blood Services routinely collects apheresis platelets from HPA-1b/1b donors and maintains a national inventory of these platelets available for urgent use in neonates with FNAIT due to anti-HPA-1a. Canadian Blood Services end labeling of platelet units indicates HPA genotypes on selected apheresis platelets and may facilitate the rapid selection of appropriate products for use in patients with FNAIT. In addition, donors with other HPA genotypes can usually be recruited expediently through the national registry to support platelet transfusion of neonates with FNAIT due to other antibodies (such as anti-HPA-5b).
Diagnostic testing for FNAIT
FNAIT is confirmed based on maternal-neonatal or maternal-paternal HPA incompatibility plus maternal alloantibodies to the specific HPA.2
Maternal HPA antibody screen
Figure 2 outlines the diagnostic workup for FNAIT, which begins with testing the mother for anti-HPA antibodies. The NPIRL employs either one of two different methods for this. The first method is an enzyme-linked immunosorbent assay (ELISA) which uses lyophilized HPAs immobilized to a well. When maternal serum is added to the well anti-HPA antibodies, if present, will bind to the well, and are then detected via a colour change. The second method is a bead-based assay whereby maternal antibodies bind to HPA coated on beads and a fluorescently labeled anti-human IgG is used to mark the beads with bound antibodies. The fluorescence is then detected on a Luminex instrument (Luminex, Austin, TX, USA). It is important to note that intravenous immunoglobulin (IVIg) will interfere with both of these testing methods. If possible, samples should be sent prior to the administration of IVIg. Regardless of which method is used, the presence or absence of anti-HPA antibodies and a presumptive antibody identification is determined. This information is generally reported within approximately three working days of the sample arriving at NPIRL.
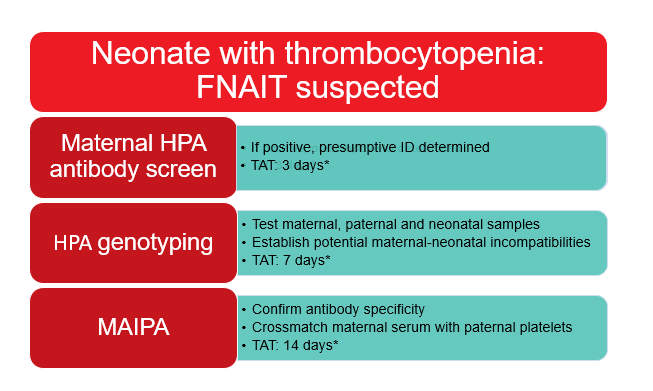
Figure 2: FNAIT workup at NPIRL.
*Turnaround times (TAT) are approximate and are counted as the number of working days from the time of sample receipt.
HPA genotyping
Regardless of the maternal HPA antibody status, HPA genotyping of maternal, paternal and neonatal samples is the next step in the diagnostic workup for FNAIT. This is accomplished using the same method for platelet donors, as described above, and allows for the identification of incompatibilities between the mother and father or neonate. The genotypes are reported within approximately seven working days of sample receipt.
MAIPA assay
The final diagnostic step is the MAIPA assay. This is a modified ELISA assay which uses monoclonal antibodies against GPIIb/IIIa and GPIa/IIa to capture antigens from lysed platelets and immobilize them to a well. The presence or absence of bound antibodies from maternal serum can then be detected by a colour change, similar to a standard ELISA.6 Depending on the mother’s antibody status and the maternal-paternal/neonatal incompatibilities identified by genotyping, either pooled platelets or specific panels of platelets with known HPA genotypes are tested against maternal serum. This serves to either confirm the specificity of an antibody or detect other reactivity that may not have been picked up on the initial screen, possibly due to a rare antibody. A MAIPA crossmatch of paternal platelets and maternal serum is also performed. The NPIRL issues a final report, including all of the MAIPA results, within approximately 14 days after sample receipt.
Given the invasive nature of fetal blood sampling, the fetal genotype is usually not available in the antenatal setting, therefore FNAIT is presumptively diagnosed on the basis of maternal and paternal samples only. Non-invasive prenatal testing using cell-free fetal DNA in maternal plasma is available for HPA-1a only and is sent out to a reference lab outside of Canada in selected high-risk cases.
Management of FNAIT
The International Collaboration for Transfusion Medicine Guidelines (ICTMG) recently published recommendations for FNAIT management based on the best available evidence.20 The key points from this document and other literature are summarized below.
Antenatal Treatment
Canada (like most other countries) does not have a routine screening program for anti-HPA antibodies in pregnant women. As such, antenatal treatment is usually implemented in women who are identified due to a previously affected pregnancy. Given that there can be severe ICH in firstborn children, a successful screening program would require very early antenatal intervention. Currently there is a lack of reliable clinical and laboratory parameters to identify fetuses with high bleeding risk and once a bleed has occurred, there is little that can be done in utero to mitigate the effects.1, 5 Furthermore, there is no current treatment to prevent development of antibodies in women known to have the HPA-1b/1b genotype, however such preventive treatments are currently under investigation.21, 22
One option for antenatal treatment is an intrauterine platelet transfusion, performed by a process similar to intrauterine red cell transfusions in HDFN. This line of treatment, however, is rarely used because of the high rate of complications (up to 11%), including fetal loss.23 Given that a fetus affected by FNAIT is already at increased risk of bleeding, instrumentation of the umbilical cord is very hazardous. In addition, the very short half-life of platelets necessitates weekly transfusions which poses practical limitations and compounds the risk of complications.5
Intravenous immunoglobulin is now considered first-line antenatal treatment for preventing thrombocytopenia or bleeding complications in FNAIT. Given the difficulty of performing randomized trials in this area, the evidence for best dosing, and timing, is lacking. The ICTMG suggests commencing weekly IVIg treatment in high-risk women at 12–16 weeks of gestation. 5, 20, 23 Corticosteroids are sometimes added to the treatment algorithm, however the evidence for their benefit is weak. 20
Postnatal Treatment
Although the evidence informing postnatal management of FNAIT is also limited, it is generally agreed that platelet transfusion should be provided without delay to a thrombocytopenic neonate. 24 Prophylactic platelet transfusion is strongly recommended to keep the platelet count over 30 x 109/l. 20 Although this threshold is somewhat arbitrary, Baker and colleagues found that the majority of ICH occurred at platelet counts less than 30 x 109/l.24 In addition, a randomized controlled trial of thrombocytopenic preterm infants showed that a liberal threshold of 50 x 109/l was associated with higher mortality and bleeding than a restrictive threshold of 25 x 109/l.25 Although neonates with FNAIT were excluded from this study, de Vos and colleagues theorized that these results may be applicable due to the common feature of fragile vasculature between preterm infants and those with FNAIT. 5
For neonates with severe bleeding, the ICTMG and other groups recommend immediate platelet transfusion to a threshold of 50 x 109/l for at least seven days.5, 20
There has also been discussion about the optimal type of platelet product that should be used to treat neonates with FNAIT; matched platelets (either HPA selected or maternal platelets), or unmatched random platelets. The ICTMG recently helped bring some clarity to this subject with a systematic review by Baker and colleagues assessing the evidence for postnatal management of FNAIT.24 The group looked at data from 754 neonates with FNAIT, 382 of whom received platelet transfusion. Of the studies that reported platelet increments and/or compared matched versus unmatched units, they found that matched platelets resulted in a higher post-transfusion increment and had a longer half-life than unmatched units (half-life of 1.9 days compared to 1 day for unmatched platelets). Despite this, the unmatched units still provided a sufficient increment to achieve hemostasis. In addition, Winkelhorst and colleagues analyzed a Dutch cohort of 102 newly diagnosed FNAIT patients and found that similar platelet increments were achieved with both HPA-matched and random pooled platelets.26
As a result of these studies, the recommendation provided by the ICTMG is to administer HPA matched platelets for prophylaxis and active bleeding in neonates with FNAIT if they are immediately available.20, 24 If not immediately available, platelet transfusion should not be delayed and unmatched units should be given, either for the entire course of treatment, or until a matched product is available.
Apart from platelet transfusion and supportive care, IVIg and/or corticosteroids are sometimes used postnatally, although there is insufficient evidence at this time to evaluate whether these are of any benefit. 24
Immunoprophylaxis
In the setting of HDFN, administration of anti-D (RhIg) to D-negative pregnant woman can prevent alloimmunization. There is interest in, and ongoing research on, developing a similar approach to prevent anti-HPA-1a development. A recombinant anti-HPA-1a antibody has been tested in humans and was found to successfully clear HPA-1a positive platelets.21, 22 HPA-1a antibodies can also be extracted from pooled donor plasma in a similar method to RhIg production. While many questions remain to be answered regarding optimal dosing, timing and efficacy, trials are ongoing and immunoprophylaxis for FNAIT may be available in the future.
Resources from Canadian Blood Services
Please see related Canadian Blood Services Customer Letters:
- Customer Letter #2020-01, HLA/HPA Results Printed on Apheresis Platelet End Label, issued January 20, 2020
- Customer Letter #2020-10, Update for HLA/HPA Results Printed on Apheresis Platelet End Label, issued March 2, 2020.
References
- Winkelhorst D, Oepkes D. Foetal and Neonatal Alloimmune Thrombocytopenia. Best Pract Res Clin Obstet Gynaecol 2019; 58: 15-27.
- Clarke G, Hannon J. Chapter 12: Hemolytic Disease of the Fetus and Newborn and Perinatal Immune Thrombocytopenia. In Clinical Guide to Transfusion. Edited by Clarke G, Chargé S. Published in Ottawa by Canadian Blood Services, 2018. https://profedu.blood.ca/en/transfusion/clinical-guide/hemolytic-disease-fetus-and-newborn-and-perinatal-immune-thrombocytopenia.
- Kjeldsen-Kragh J, Killie MK, Tomter G, Golebiowska E, Randen I, Hauge R, Aune B, Oian P, Dahl LB, Pirhonen J, Lindeman R, Husby H, Haugen G, Gronn M, Skogen B, Husebekk A. A Screening and Intervention Program Aimed to Reduce Mortality and Serious Morbidity Associated with Severe Neonatal Alloimmune Thrombocytopenia. Blood 2007; 110: 833-9.
- Kamphuis MM, Paridaans N, Porcelijn L, De Haas M, Van Der Schoot CE, Brand A, Bonsel GJ, Oepkes D. Screening in Pregnancy for Fetal or Neonatal Alloimmune Thrombocytopenia: Systematic Review. Bjog 2010; 117: 1335-43.
- de Vos TW, Winkelhorst D, de Haas M, Lopriore E, Oepkes D. Epidemiology and Management of Fetal and Neonatal Alloimmune Thrombocytopenia. Transfus Apher Sci 2019: 102704.
- Metcalfe P. Platelet Antigens and Antibody Detection. Vox Sang 2004; 87 Suppl1: 82-6.
- McQuilten ZK, Wood EM, Savoia H, Cole S. A Review of Pathophysiology and Current Treatment for Neonatal Alloimmune Thrombocytopenia (Nait) and Introducing the Australian Nait Registry. Aust N Z J Obstet Gynaecol 2011; 51: 191-8.
- Winkelhorst D, Kamphuis MM, de Kloet LC, Zwaginga JJ, Oepkes D, Lopriore E. Severe Bleeding Complications Other Than Intracranial Hemorrhage in Neonatal Alloimmune Thrombocytopenia: A Case Series and Review of the Literature. Transfusion 2016; 56: 1230-5.
- Tiller H, Kamphuis MM, Flodmark O, Papadogiannakis N, David AL, Sainio S, Koskinen S, Javela K, Wikman AT, Kekomaki R, Kanhai HH, Oepkes D, Husebekk A, Westgren M. Fetal Intracranial Haemorrhages Caused by Fetal and Neonatal Alloimmune Thrombocytopenia: An Observational Cohort Study of 43 Cases from an International Multicentre Registry. BMJ Open 2013; 3.
- Newman PJ, Derbes RS, Aster RH. The Human Platelet Alloantigens, Pla1 and Pla2, Are Associated with a Leucine33/Proline33 Amino Acid Polymorphism in Membrane Glycoprotein Iiia, and Are Distinguishable by DNA Typing. J Clin Invest 1989; 83: 1778-81.
- Ohto H, Miura S, Ariga H, Ishii T, Fujimori K, Morita S. The Natural History of Maternal Immunization against Foetal Platelet Alloantigens. Transfus Med 2004; 14: 399-408.
- 1Liu ZJ, Bussel JB, Lakkaraja M, Ferrer-Marin F, Ghevaert C, Feldman HA, McFarland JG, Chavda C, Sola-Visner M. Suppression of in Vitro Megakaryopoiesis by Maternal Sera Containing Anti-Hpa-1a Antibodies. Blood 2015; 126: 1234-6.
- Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, Jackson CW, Hunt P, Saris CJ, Orkin SH. Transcription Factor Nf-E2 Is Required for Platelet Formation Independent of the Actions of Thrombopoietin/Mgdf in Megakaryocyte Development. Cell 1995; 81: 695-704.
- Yougbare I, Zdravic D, Ni H. Angiogenesis and Bleeding Disorders in Fnait. Oncotarget 2015; 6: 15724-5.
- van Gils JM, Stutterheim J, van Duijn TJ, Zwaginga JJ, Porcelijn L, de Haas M, Hordijk PL. Hpa-1a Alloantibodies Reduce Endothelial Cell Spreading and Monolayer Integrity. Mol Immunol 2009; 46: 406-15.
- Santoso S, Wihadmadyatami H, Bakchoul T, Werth S, Al-Fakhri N, Bein G, Kiefel V, Zhu J, Newman PJ, Bayat B, Sachs UJ. Antiendothelial Alphavbeta3 Antibodies Are a Major Cause of Intracranial Bleeding in Fetal/Neonatal Alloimmune Thrombocytopenia. Arterioscler Thromb Vasc Biol 2016; 36: 1517-24.
- Kumpel BM, Sibley K, Jackson DJ, White G, Soothill PW. Ultrastructural Localization of Glycoprotein Iiia (Gpiiia, Beta 3 Integrin) on Placental Syncytiotrophoblast Microvilli: Implications for Platelet Alloimmunization During Pregnancy. Transfusion 2008; 48: 2077-86.
- Kjeldsen-Kragh J, Skogen B. Mechanisms and Prevention of Alloimmunization in Pregnancy. Obstet Gynecol Surv 2013; 68: 526-32.
- Williamson LM, Hackett G, Rennie J, Palmer CR, Maciver C, Hadfield R, Hughes D, Jobson S, Ouwehand WH. The Natural History of Fetomaternal Alloimmunization to the Platelet-Specific Antigen Hpa-1a (Pla1, Zwa) as Determined by Antenatal Screening. Blood 1998; 92: 2280-7.
- Lieberman L, Greinacher A, Murphy MF, Bussel J, Bakchoul T, Corke S, Kjaer M, Kjeldsen-Kragh J, Bertrand G, Oepkes D, Baker JM, Hume H, Massey E, Kaplan C, Arnold DM, Baidya S, Ryan G, Savoia H, Landry D, Shehata N. Fetal and Neonatal Alloimmune Thrombocytopenia: Recommendations for Evidence-Based Practice, an International Approach. Br J Haematol 2019; 185: 549-62.
- Ghevaert C, Wilcox DA, Fang J, Armour KL, Clark MR, Ouwehand WH, Williamson LM. Developing Recombinant Hpa-1a-Specific Antibodies with Abrogated Fcgamma Receptor Binding for the Treatment of Fetomaternal Alloimmune Thrombocytopenia. J Clin Invest 2008; 118: 2929-38.
- Ghevaert C, Herbert N, Hawkins L, Grehan N, Cookson P, Garner SF, Crisp-Hihn A, Lloyd-Evans P, Evans A, Balan K, Ouwehand WH, Armour KL, Clark MR, Williamson LM. Recombinant Hpa-1a Antibody Therapy for Treatment of Fetomaternal Alloimmune Thrombocytopenia: Proof of Principle in Human Volunteers. Blood 2013; 122: 313-20.
- Winkelhorst D, Murphy MF, Greinacher A, Shehata N, Bakchoul T, Massey E, Baker J, Lieberman L, Tanael S, Hume H, Arnold DM, Baidya S, Bertrand G, Bussel J, Kjaer M, Kaplan C, Kjeldsen-Kragh J, Oepkes D, Ryan G. Antenatal Management in Fetal and Neonatal Alloimmune Thrombocytopenia: A Systematic Review. Blood 2017; 129: 1538-47.
- Baker JM, Shehata N, Bussel J, Murphy MF, Greinacher A, Bakchoul T, Massey E, Lieberman L, Landry D, Tanael S, Arnold DM, Baidya S, Bertrand G, Kjaer M, Kaplan C, Kjeldsen-Kragh J, Oepkes D, Savoia H, Ryan G, Hume H. Postnatal Intervention for the Treatment of Fnait: A Systematic Review. J Perinatol 2019; 39: 1329-39.
- Curley A, Stanworth SJ, Willoughby K, Fustolo-Gunnink SF, Venkatesh V, Hudson C, Deary A, Hodge R, Hopkins V, Lopez Santamaria B, Mora A, Llewelyn C, D'Amore A, Khan R, Onland W, Lopriore E, Fijnvandraat K, New H, Clarke P, Watts T. Randomized Trial of Platelet-Transfusion Thresholds in Neonates. N Engl J Med 2019; 380: 242-51.
- Winkelhorst D, Oostweegel M, Porcelijn L, Middelburg RA, Zwaginga JJ, Oepkes D, van der Bom JG, de Haas M, Lopriore E. Treatment and Outcomes of Fetal/Neonatal Alloimmune Thrombocytopenia: A Nationwide Cohort Study in Newly Detected Cases. Br J Haematol 2019; 184: 1026-9.