FAQ: Canadian Blood Services platelet bacterial screening
Authors: Aditi Khandelwal, MDCM, FRCPC; Sandra Ramirez-Arcos, MSc, PhD; and Mark Bigham, MD, MHSc, FRCPC
Primary target audiences: Medical laboratory technologists (MLTs) in a hospital laboratory, transfusion medicine physicians
Introduction
At Canadian Blood Services, buffy coat pooled platelet and apheresis platelet units that have not been treated with psoralen (untreated platelet units), undergo routine bacterial screening early in their shelf life. Routine bacterial screening of platelet components was implemented in 2004. More recently, in 2017, implementation of a bacterial screening program extended the shelf life of untreated platelet units from 5 to 7 days.
This FAQ will help hospital customers better understand the current reporting of positive bacterial testing results and its implications for both the hospital customer and Canadian Blood Services (Figure 1).
As of 2022, psoralen-treated (pathogen-reduced) platelet units are also available in select regions of Canada. As these platelet units are pathogen-reduced, they do not undergo routine bacterial screening early in their shelf-life. However, as part of a quality control sterility program, 1% of pathogen-reduced platelet units will still undergo bacterial screening at expiry as required by the Canadian Standards Association.
More information on Canadian Blood Services’ platelet products is available here:
- Chapter 2, Blood Components
- Chapter 6, Donor Selection, Donor Testing and Pathogen Reduction
- Chapter 18, Platelet Transfusion, Alloimmunization and Management of Platelet Refractoriness
- Canadian Blood Services Circular of Information, Pooled Platelets LR CPD, Apheresis Platelets1
- Canadian Blood Services Circular of Information, Pooled Platelets Psoralen Treated2
Frequently Asked Questions
When is bacterial screening performed on platelet units?
As part of the production process, untreated platelet units are quarantined for a minimum of 36 hours before samples are drawn for both aerobic and anaerobic bacterial testing. Samples for bacterial screening are collected from every untreated platelet unit and are inoculated into BACT/ALERT aerobic and anaerobic culture bottles. Culture bottles are incubated for 7 days in the BACT/ALERT 3D system (BACT). After sampling, the untreated platelet units are further quarantined for a minimum of 6 hours.
Are untreated platelet units that test positive for bacterial culture released to hospitals?
Untreated platelet units are issued to hospitals if the BACT culture is “negative to date” at the time of issue. Hence, platelet units will be issued before the end of the 7-day culture.
If the BACT culture becomes positive after the platelet unit is issued (“initial positive” bacterial culture), the hospital is notified, and a precautionary recall of all blood components associated with the same collection(s) is initiated from all hospitals.
If a BACT culture is initial positive, is this result confirmed with additional tests?
If there is an initial positive result in a BACT culture bottle, confirmatory testing is performed on the index platelet unit if it is available. If the index platelet unit is not available, confirmatory testing is done on the associated red blood cell concentrates (only for pooled platelets). A platelet unit is considered “confirmed positive” when the same bacteria detected in the initial positive culture is identified in confirmatory test(s).
For platelet units that have been recalled due to an initial positive BACT culture, what is the risk that they are truly contaminated?
Canadian Blood Services data obtained between August 2017 to December 2020 indicate that only 0.10% buffy coat platelet units and 0.04% apheresis platelet units were confirmed as true bacterial contaminations. During that same time frame, for platelet units that have been recalled due to an initial positive BACT culture, 27.9% buffy coat platelet units and 5.5% apheresis platelet units were confirmed to be positive for bacterial contamination. The majority of identified bacterial species were skin commensal bacteria.3 The most recent results can be found in Canadian Blood Services’ annual surveillance report.
If a hospital customer receives notification of a platelet unit recall due to positive bacterial culture, what are the recommended next steps?
Following hospital receipt of a platelet unit recall, if the recalled unit was transfused, it is recommended that the patient’s clinical status and health record be reviewed to assess for evidence of possible bacterial transfusion-transmitted infection (TTI).
The hospital Transfusion Medicine Director should also be consulted for advice regarding clinical management and reporting. Clinical signs of a bacterial TTI may include fever, chills, rigors, nausea, vomiting, diarrhea, abdominal and muscle pain, hypotension, hemoglobinuria, disseminated intravascular coagulation and/or renal failure. Other clinical criteria of a possible bacterial TTI are outlined in the Public Health Agency of Canada (PHAC) Guideline for Investigation of Suspected Transfusion Transmitted Bacterial Contamination.4 If there is no evidence of bacterial sepsis, then no further action (including recipient notification) is recommended.5 If the patient’s clinical status suggests a possible bacterial TTI, then steps outlined in the PHAC Guideline to investigate for bacterial TTI should be undertaken, recognizing that pre-transfusion bacterial infection or antibiotic treatment can complicate the assessment.
Suspected bacterial TTI should be reported to Canadian Blood Services using the recommended transfusion reaction report form for your jurisdiction. For guidance on reporting adverse transfusion reactions, including links to reporting forms, see A Guide to Reporting Adverse Transfusion Reactions.
Once an initial positive bacterial culture is identified, what steps are undertaken by Canadian Blood Services?
Following an initial positive bacterial culture, Canadian Blood Services conducts additional testing of the positive platelet culture and of the original component and companion components (if applicable/available). The testing is performed at a Canadian Blood Services laboratory. The timeline for this additional testing by Canadian Blood Services may extend to several weeks after the product recall. Although additional testing results are not intended for patient clinical management, Canadian Blood Services will report results of follow-up Gram stain and bacterial identification to hospitals as soon as available for all associated transfused components.
Questions about follow-up results may be directed to the hospital Transfusion Medicine Director or to a Canadian Blood Services Medical Officer.
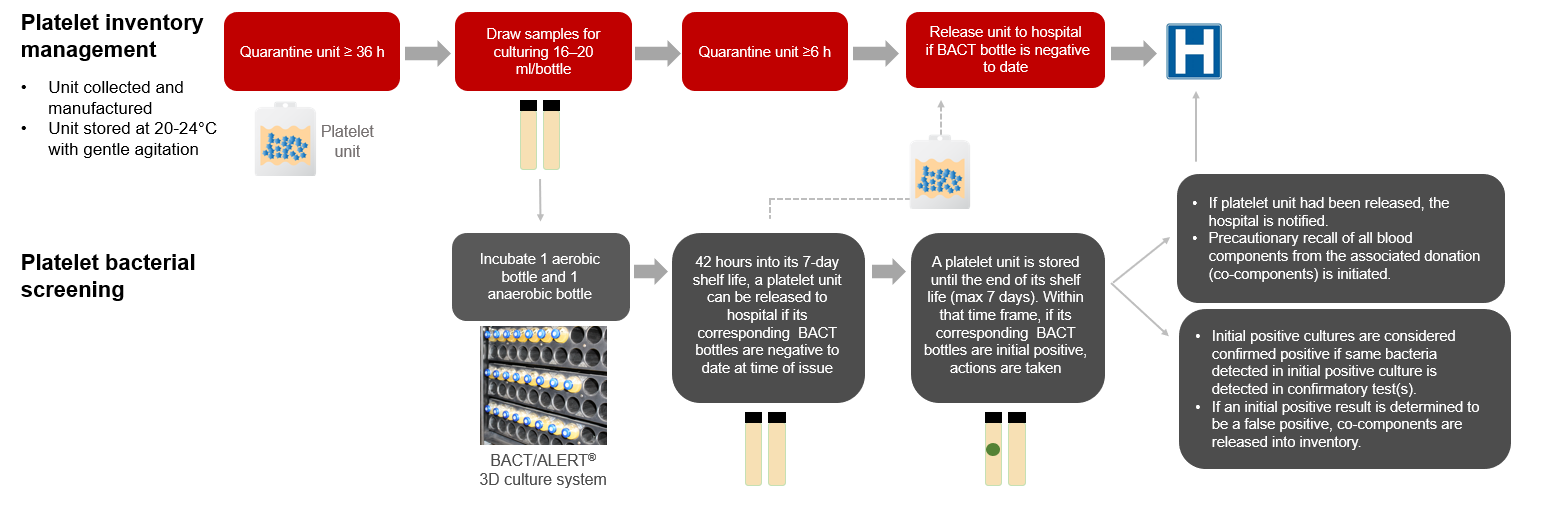
Figure 1: Untreated platelet bacterial testing at Canadian Blood Services.
References
-
Canadian Blood Services. Circular of Information, Pooled Platelets LR CPD, Apheresis Platelets, 2022. https://www.blood.ca/en/hospital-services/products/component-types/circular-information (Last accessed September 12 2022).
-
Canadian Blood Services. Circular of Information Pooled Platelets Psoralen Treated, 2022. https://www.blood.ca/en/hospital-services/products/component-types/circular-information (Last accessed September 12 2022).
-
Ramirez-Arcos S, Evans S, McIntyre T, Pang C, Yi Q-L, DiFranco C, Goldman M. Extension of Platelet Shelf Life with an Improved Bacterial Testing Algorithm. Transfusion 2020; 60: 2918-28. https://onlinelibrary.wiley.com/doi/abs/10.1111/trf.16112.
-
Public Health Agency of Canada. Guideline for Investigation of Suspected Transfusion Transmitted Bacterial Contamination, 2007. https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2008-34/guideline-investigation-suspected-transfusion-transmitted-bacterial-contamination.html (Last accessed February 24 2021).
-
National Advisory Committee on Blood and Blood Products and Canadian Blood Services. Recommendations for the Notification of Recipients of a Blood Component Recall. National Advisory Committee on Blood and Blood Products, 2015. http://www.nacblood.ca/resources/guidelines/recall-recipient-notification.html.