Chapter 11
Massive hemorrhage and emergency transfusion
Background
The approach to transfusion in emergent situations varies dramatically depending on the clinical scenario. The clinician’s assessment of the rapidity of bleeding, the severity of hemorrhage or amount of blood lost, and the clinical stability of the patient will determine the transfusion strategy.
Hemodynamically stable patients with slow to moderate bleeding, chronic bleeding, or contained blood loss can usually be managed conservatively with crystalloid to maintain intravascular volume, when necessary. In these patients, transfusion decisions should be guided by the clinical status of the patient. Numerous studies have established that a restrictive (70 to 80 g/L) red blood cell transfusion strategy is superior or equivalent to a liberal strategy (90 to 100 g/L) in critically ill adult1 and pediatric2 medical patients, gastrointestinal (GI) bleeding3, traumatic brain injury4, septic shock5, elderly orthopedic6 and cardiac surgery patients.7-9 Hemodynamically stable, non-bleeding patients exhibiting symptoms of inadequate oxygen delivery should be transfused one red blood cell unit at a time and reassessed.
Hemodynamically unstable patients with rapid bleeding require a completely different approach. Management of massive hemorrhage, as seen with severely injured trauma or obstetrical patients, has changed dramatically over the past 10 years. The recognition that trauma patients are often profoundly coagulopathic at the time of presentation has refocused energy and clinical research into redefining how these patients are managed. Using the severely injured trauma patient as a case study, this chapter will discuss the principles of massive hemorrhage and resuscitation, with the inclusion of special situations such as obstetrical hemorrhage, where data are available. Extension of practices from the trauma literature to other bleeding critically ill patients is not always appropriate10, 11; however, most clinical studies and experience have come from trauma patients, and many general principles of emergency transfusion and bleeding management apply.
Successful management of massive hemorrhage requires a coordinated, pre-planned effort that involves the entire care team. It is ideally guided by an institution-specific protocol that incorporates all of the basic principles for the management of rapidly bleeding patients.
Establishing an institutional protocol for management of massive hemorrhage: the massive hemorrhage protocol (MHP)
Massive hemorrhage protocols (MHPs) are tools designed to expedite the provision of blood components based on best practices for the management of massively bleeding patients. These best practices include: early identification of the massively bleeding patient, rapid provision of blood components (red blood cells, platelets, plasma) or blood products (plasma protein and related products), or both, to the bedside, and a coordinated human resource response aimed at rapid identification and control of bleeding.
The terms “massive hemorrhage protocol” and “massive transfusion protocol” (MTP) are both used in the literature. MHP is used in this chapter as it better reflects goals that extend beyond transfusion of blood products to also include hemorrhage control and other important non-transfusion interventions.12
The implementation of an MHP for trauma is associated with improved patient outcomes, less overall blood utilization, and cost savings.13-17 In trauma, each 1-minute delay in the provision of blood components and products to the bedside is associated with a 5% increase in mortality.18 It is generally accepted that implementation of an MHP improves the care of patients with other forms of severe hemorrhage, including post-partum hemorrhage, but this remains to be definitively shown in the literature.
Development of an MHP is ideally a multidisciplinary process that accounts for local practice, local inventory, logistics, human resource availability, and system limitations. Bedside clinicians (e.g., anesthesiologists, trauma surgeons, emergency physicians, obstetricians, nurses), transfusion medicine specialists (e.g., hematologists, pathologists, hematopathologists), blood bank and laboratory representatives, and allied care staff (e.g., porters, communications specialists) should all be included in the MHP development process to ensure that the MHP is appropriate for, and executable at, that team’s institution. A specific protocol, in turn, may not be generalizable to all sites. An effective MHP engages everyone on the clinical team, encourages communication with standardized language both between and within the clinical and laboratory teams, and standardizes transfusion best practices. The establishment of clear protocols for blood management, bedside testing, and transfusion decision-making decreases cognitive load and allows practitioners to focus on other aspects of patient care.
All hospitals with emergency departments, operating rooms, or an obstetrical service should have an MHP. Processes for initiating and terminating the MHP need to be in place. In the context of a limited blood supply and/or a code orange scenario, hospitals should communicate early with the blood centre. Tertiary and quaternary care centres within a health authority/province often have established MHPs that can be modified and adopted for smaller centres. Up-to-date and highly practical Canadian-specific guidance and tools for MHP development can be accessed on the Ontario Regional Blood Coordinating Network (ORBCoN) website. Finally, care teams are encouraged to debrief all MHP activations as an opportunity to improve MHP delivery, and the MHP itself should be reviewed regularly to ensure that it continues to incorporate best practices.
Identification of massive hemorrhage
Early recognition and identification of the patient who will require a massive transfusion is critical to successful resuscitation. Many definitions of massive transfusion exist, such as the replacement of one blood volume (or more) in 24 hours, 10 or more units of red blood cells in 24 hours, or replacement of more than 50% blood volume in four hours.19 Unfortunately, these definitions are retrospective and not helpful when faced with a bleeding patient. Other definitions may be more clinically useful, such as the “critical administration threshold,” defined as a requirement for ≥3 red blood cell units in one hour.20
In addition, objective patient assessment using a validated risk assessment tool is helpful, as clinical judgement alone has both poor sensitivity and specificity (~65%).21 Many tools are available, using a combination of clinical assessment, laboratory values, and bedside ultrasonography [Focused Assessment with Sonography for Trauma (FAST)].22 Though scores using all three elements are most predictive, the complexity of these tools and the potential delay from laboratory result turnaround time limit their practical use. The shock index (SI), calculated by dividing heart rate (HR) by systolic blood pressure (SBP), is recommended to assess degree of hypovolemic shock.23 Additional considerations, such as mechanism of injury and ultrasound assessment, increase the sensitivity for predicting an MHP requirement. Examples of validated tools to trigger an MHP are illustrated in Figure 1.
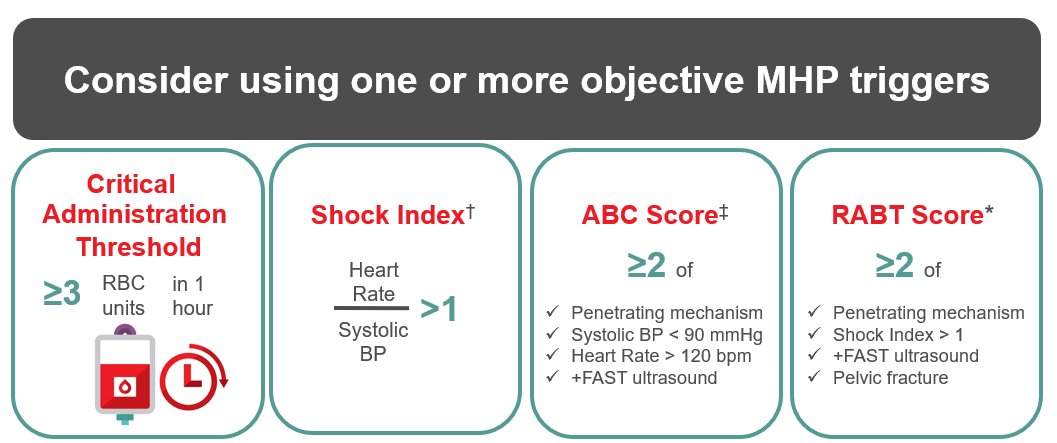
† Shock Index (SI) > 1 after ≥1 L of fluid is 48% sensitive and 91% specific for prediction of MHP requirement.22
‡ Assessment of Blood Consumption (ABC) score ≥2 is 75% sensitive and 86% specific for prediction of MHP requirement.23
* Revised Assessment of Bleeding and Transfusion (RABT) score ≥2 is 78% sensitive and 91% specific for prediction of MHP requirement.24
Figure 1: Validated, objective measures for triggering an MHP
Trauma-induced coagulopathy (TIC)
In severely injured trauma patients, exsanguinating hemorrhage is the most common cause of death in the first hour, and accounts for 50% of deaths in the first 24 hours.24 Historically, it was assumed that trauma patients became coagulopathic during the course of their resuscitation due to dilution, depletion, and dysfunction of procoagulant factors as they received progressively more crystalloid and became increasingly cold and acidemic. It has now been well established that a significant proportion (25–40%) of severely injured trauma patients are already coagulopathic at the time of presentation to hospital, and that this coagulopathy is associated with an increased risk of mortality.25 Trauma-induced coagulopathy (TIC) is characterized by endothelial dysfunction, dysfibrinogenemia, platelet dysfunction and an imbalance of procoagulant and anticoagulant factors with systemic anticoagulation. This process is exacerbated by hypothermia, acidemia, resuscitation with hypocoagulable fluids, hypoperfusion, and ongoing bleeding with further consumption of coagulation factors.26
The recognition that TIC presents very early has dramatically changed the approach to the severely injured trauma patient and, by extension, the management of massive bleeding in other populations. Attempts to ameliorate the coagulopathy of acute trauma have led to the development and proliferation of MHPs, a focus on damage control resuscitation, and ratio-driven resuscitation with an emphasis on early provision of plasma, platelets and procoagulant factors. The appropriateness of whether trauma resuscitation principles should extend to non-trauma populations will be revisited later in the chapter.
Damage control resuscitation (DCR)
Damage control resuscitation (DCR) comprises a set of resuscitation principles with the goal of arresting or limiting TIC and the physiologic consequences associated with resuscitation.27, 28 It applies only to the most seriously injured trauma patients that are approaching physiologic exhaustion. DCR is defined by the employment of four simultaneous strategies:
1. Early hemorrhage control in the form of damage control surgery or interventional radiology. When the risk of ongoing shock and TIC is high, limit surgical intervention and delay definitive management in order to restore metabolic homeostasis and reverse coagulopathy.
2. Avoidance or strict limitation of crystalloid use. Traditional trauma resuscitation was initiated with large volumes of crystalloid followed by 6 to 10 units of red blood cells, prior to consideration of other blood products. Large volume crystalloid resuscitation is no longer recommended because it exacerbates coagulopathy and is associated with several deleterious side effects, including tissue edema, acidosis, reperfusion injury and multiorgan failure.29, 30 Recognizing this, best practice recommended in the current Advanced Trauma Life Support® (ATLS®) guidelines suggest limiting crystalloid use to 1 litre prior to switching to blood products.31
3. Limit hemorrhage. The concept of “permissive hypotension,” allowing a patient’s blood pressure to remain below normal (mean arterial pressure of 50–60 mmHg), is a prehospital strategy that is used to limit ongoing hemorrhage in the absence of suspected brain injury. In hospital, it makes sense to target normal blood pressure to ensure adequate perfusion of critical organs such as the brain, heart and kidneys. This is particularly important in the brain or spinal cord injured patient, where higher mean arterial pressure (≥80 mmHg) is recommended.32
4. Massive hemorrhage protocol. Employment of an MHP that provides rapid delivery of predetermined, fixed ratio blood components and products to the bedside. Modern MHPs aim to provide blood component therapy in a ratio that approximates whole blood (i.e., one unit of plasma: one unit of platelets: one unit of red blood cells). Early military studies demonstrated a significant survival benefit to soldiers with severe traumatic injury receiving plasma and platelets in addition to red blood cells during the early phase of resuscitation.33 The Pragmatic Randomized Optimized Platelet and Plasma Ratios (PROPPR) trial is the most recent multicentre, prospective randomized trial aimed to definitively answer this question in civilian trauma patients. There was no overall survival benefit demonstrated with provision of a 1:1:1 blood component strategy as compared to a 1:1:2 strategy of one unit of plasma: one unit of platelets: two units of red blood cells. A secondary outcome showed a reduction in bleeding and exsanguination in the first 24 hours.28
At the moment, the optimal ratio of components and products is unknown. We know that early, aggressive resuscitation with blood components, rather than crystalloid, and early introduction of non-red blood cell components in a relatively balanced approach can limit the extent of TIC and its associated mortality. These principles are also used to guide the resuscitation of less severely injured trauma patients and often massively bleeding non-trauma patients. However, caution must be exercised when extending what is learned from resuscitating the trauma patient to the non-trauma patient, as they are likely to have different mechanisms of injury, and thus different pathophysiology of tissue injury and coagulopathy. The few studies available suggest that the aggressive 1:1:1 (or near 1:1:1) transfusion approach is not necessary in non-traumatic bleeding such as that seen in gastrointestinal (GI) bleeds or perioperative bleeding.34, 35 In these populations, an aggressive 1:1:1 resuscitation strategy is likely not necessary, and in some studies has been associated with harm.34, 35 For each of these scenarios, the ideal transfusion strategy is not known.
For resuscitation of the unstable bleeding non-trauma, non-obstetrical patient, it is reasonable to start with red blood cell transfusion. Consideration of plasma, platelets and/or fibrinogen supplementation will depend more on early and frequent assessment of coagulation parameters, including fibrinogen concentration and platelet count. The frequency of measurement will depend on the rapidity of bleeding and availability of resources. Once hourly is a reasonable starting point for most bleeding patients. A careful medication history is also required, to account for the presence of platelet inhibiting medications or other anticoagulants.
Resuscitation of the massively bleeding patient is not easy. There are many factors that contribute to the confusion and complexity: patient factors, human resources, practitioner availability and bias, hospital and blood bank resources, and system factors. The development and successful implementation of a protocol to manage bleeding patients is likely one of the most meaningful ways to have an effective impact on patient outcomes.
Required adjuncts for management of the bleeding patient
Tranexamic acid (TXA)
Early provision of tranexamic acid (TXA) to the traumatically injured patient improves outcomes. The largest study of TXA in trauma patients (CRASH-2) revealed that TXA administration improved both all-cause mortality and mortality related to bleeding. Benefit was also seen in patients at risk for bleeding, in whom subsequent “massive transfusion” was not required. Follow-up analyses of the CRASH-2 study have demonstrated that the benefit occurred when TXA was administered within three hours of injury. Based on these findings, most jurisdictions have included TXA administration in their prehospital algorithm or early in their MHP for all trauma patients deemed at risk for bleeding.36, 37
TXA has also been shown to decrease death due to bleeding in postpartum hemorrhage,38 and reduce bleeding in cardiac surgery,39 orthopedic surgery,40 spine surgery41 and improve outcomes in mild to moderate traumatic brain injury.42
Fibrinogen supplementation
Fibrinogen is critically important for hemostasis. In the massively bleeding trauma patient, fibrinogen levels fall to critically low levels early after injury.43 Multiple studies have demonstrated increased bleeding risk and poorer outcomes in patients with low fibrinogen levels in trauma,44 cardiac surgery45 and postpartum hemorrhage.46 Contemporary appreciation of the importance of adequate fibrinogen replacement in bleeding patients is reflected by recent changes to many guidelines recommending a fibrinogen level of at least 1.5–2.0 g/L in the context of bleeding,47, 48 with higher, as yet undefined targets in the context of postpartum hemorrhage.49 Evidence that early fibrinogen replacement improves survival in trauma patients exists,50, 51 and ongoing randomized controlled trials (RCTs) will help to define the importance of early fibrinogen supplementation in trauma.52-55 In Canada, fibrinogen replacement should occur with fibrinogen concentrate or cryoprecipitate. A 2019 multicentre RCT in cardiac surgery demonstrated the equivalent efficacy and safety of fibrinogen concentrate and cryoprecipitate.56 This study resulted in the Health Canada approval of fibrinogen concentrate for the management of hypofibrinogenemia in bleeding patients.
Although either cryoprecipitate or fibrinogen concentrate can be utilized for fibrinogen replacement therapy, the concentrate has the advantage of being pathogen-reduced and available in a freeze-dried, powdered form, which makes it easier to reconstitute and administer than cryoprecipitate, which must remain frozen until use. For these reasons, fibrinogen concentrate is increasingly used in Canada for treatment of acquired hypofibrinogenemia (see Chapter 2, Blood Components).
Of utmost importance is to remember to test fibrinogen levels and replace it early in massively bleeding patients.
Laboratory Testing Capability
Ideally, baseline laboratory tests are drawn at presentation (CBC, INR, PTT, fibrinogen, arterial or venous blood gas, lactate, electrolytes, and ionized calcium), and at a minimum of one hour intervals12, to guide transfusion decisions, and to monitor for, and correct metabolic abnormalities. Testing capability and turn around time will vary by site.
Appropriate selection of components for emergency transfusion
Clinical assessment of the urgency for red blood cell transfusion will determine whether the patient receives uncrossmatched emergency type O red blood cells, group-specific red blood cells, or a fully crossmatched red blood cell unit. In all cases, a pre-transfusion sample of appropriately identified and labelled blood should be obtained from the patient and sent to the blood bank for typing and initiation of compatibility testing (see Chapter 8, Pre-transfusion Testing). Risks of potentially fatal ABO transfusion errors are high in urgent clinical situations involving trauma patients. Particular care and attention must accompany patient identification procedures in this setting.
Type O uncrossmatched red blood cells should be used if the patient's blood group is unknown and transfusion is immediately required. In this scenario, type O Rh-positive red blood cells can be transfused to males who have no prior history of transfusion with Rh-positive blood. Type O Rh-negative red blood cells should be reserved for females of childbearing age,57 children, and others suspected or known to be alloimmunized to the D antigen.58 Type-specific uncrossmatched blood can usually be provided within 15 minutes; however, completion of an antibody screen and crossmatch often takes 30–60 minutes. In the setting of an emergency transfusion that is initiated with emergency supply type O blood, a switch to group-specific product should happen as soon as the patient’s blood type is known and ABO verification is complete (via a second determination of the recipient’s blood group, see CSA standard CAN/CSA-Z902:20, Blood and blood components 10.6.1.3)59 regardless of the number of type O units the patient has received. Transfusing physicians should familiarize themselves with the policies and procedures of their local hospital blood bank in providing blood for emergency use.
Risks and complications associated with large volume resuscitation with blood components and products
1. Hypothermia
Massive transfusion can easily result in clinically significant hypothermia (body temperature below 35°C). Hypothermia dramatically worsens platelet and coagulation function, decreases citrate metabolism, increases hemoglobin-oxygen affinity (decreasing oxygen release to the tissues), and decreases myocardial function. Aggressive temperature management is imperative to successful treatment during massive transfusion. This can be accomplished by warming of the resuscitation bay or operating room, infusion of all products through an approved blood warming device, and employment of external warming devices. The patient’s temperature should be actively monitored (continuously, or at a minimum of hourly). Precautions for avoidance of air embolism must be considered with the use of pressurized infusion systems.
2. Impaired hemostasis
Patients who present with trauma or tissue injury can have significant coagulation defects at presentation. This coagulopathy is exacerbated by resuscitation with both crystalloid and starches (e.g., hydroxyethyl starch). Large volume resuscitation with blood components is more favourable but dilution of platelets and clotting factors, particularly fibrinogen, can still occur. In the early phases of resuscitation when bleeding is rapid, transfusion should be guided by the institution’s MHP, with replacement of blood components (red blood cell, plasma, and platelets) in a predetermined ratio, with early consideration for the addition of fibrinogen replacement (fibrinogen concentrate or cryoprecipitate). As time permits, transfusion can then be guided by either standard laboratory tests and/or viscoelastic tests of whole blood clotting (ROTEM® or TEG®).
3. Hypocalcemia and citrate toxicity
Blood components are anticoagulated with sodium citrate. Transfused citrate binds calcium and magnesium, and suboptimal citrate metabolism in the context of massive transfusion may lead to citrate toxicity and hypocalcemia. Hypocalcemia can lead to hypotension, impaired coagulation, reduced ventricular function and increased neuromuscular excitability. Calcium monitoring and replacement are essential and must be included in the MHP.
4. Hyperkalemia
Potassium leaks from red blood cells during storage and can reach levels of up to 80 mmol/L in a red blood cell unit. In rare cases, hyperkalemia can result in cardiac arrhythmias, myocardial depression, or cardiac arrest.
5. Volume overload/over transfusion
Massively transfused patients, particularly those with ongoing hemorrhage, are vulnerable to extremes of intravascular volume (hypovolemia to hypervolemia) and myocardial depression. Physical examination may be inadequate to guide management of these patients; invasive monitoring methods (central venous pressure, pulmonary artery catheter, transesophageal echocardiography) may be required.
6. Alloimmunization and delayed hemolytic transfusion reaction
Especially in cases where uncrossmatched blood is used, there is a risk of alloimmunization. Development of red blood cell antibodies to foreign antigens (alloimmunization) puts female patients at risk for future hemolytic disease of the fetus or newborn, renders a patient more difficult to crossmatch in the future, increases the risk of transfusion reactions, and complicates matching for solid organ transplantation. The seroconversion rate for RhD- trauma patients of childbearing age receiving O+ emergency supply blood can be as high as 50%.60, 61 While the clinical consequence of seroconversion in males and females past child-bearing age is low, this underscores the need for provision of O- emergency supply blood to females 45 years and younger. Similarly, Kell negative blood is now used in Canada for transfusion to women of childbearing age, and should be maintained in trauma situations.2
The risk of delayed hemolytic transfusion reaction (DHTR) exists in the context of transfusing uncrossmatched blood to a patient with unidentified RBC alloantibodies. The prevalence of alloantibodies in patients that present to hospital is 1–3%, and the presence of clinically significant antibodies is approximately 1%. Antibody incidence is higher in multiparous females and increases with age.63 However, the risk of a clinically significant delayed hemolytic transfusion reaction in a series of transfused trauma patients is very low, in the order of 0.02%.64, 65
Emerging Practices and Products
Inconsistency in massive hemorrhage protocol-based resuscitation persists, as evidence-based best practice is difficult to conclusively study. Lack of consensus reflects the tension between requiring aggressive therapy in critically ill patients, ensuring that therapy minimizes harm, and balancing the logistics of blood supply and human resources. A number of practices are beginning to gain prominence, though they do not necessary reflect standard of care across Canada and other jurisdictions.
Prehospital transfusion
With the notion that even minutes of delay lead to increased mortality in MHPs,18 resuscitation with blood components and plasma protein and related products have emerged in the prehospital space, usually during air transport. It is a significant challenge to create programs that ensure transfusion medicine safety and accreditation standards are met, that minimize unnecessary wastage, and are able to maintain competencies of staff. Though the vast majority of prehospital transfusion programs utilize red blood cells66 and tranexamic acid, the only RCTs in prehospital transfusion have been done using plasma transfusion.67, 68 Though evidence is conflicting, factors that may produce favourable patient outcomes with prehospital transfusion include longer injury-to-arrival times,69 co-administration of red blood cells and plasma,70 and patients with blunt injury.71 Above all, prompt transportation to a centre that can provide definitive care is paramount.
Alternatives to AB plasma
Group AB plasma transfusion has been increasing as a proportion of overall plasma use, given its popularity for emergency release in MHPs. Unfortunately, group AB plasma donors represent only 4% of total blood donors. Two emerging practices that address alternatives to group AB plasma include: (1) clotting factor concentrates such as a combination of prothrombin complex concentrate (PCC, 2000 IU) and fibrinogen concentrate (FC 4 grams) is suggested in lieu of plasma availability and can be feasibly implemented in remote settings12,51; and (2) group A plasma for emergency transfusion when the patient’s blood group is unknown. Use of group A plasma is standard of care in many trauma centres in the U.S., with two large retrospective studies supporting its safety.72, 73
Whole blood and other component alternatives
Blood components and products that were used in the early days of transfusion practice, including freeze dried plasma, cold-stored platelets, and cold-stored low-titre group O whole blood (LTOWB), are increasingly being used again in bleeding patients. The use of LTOWB may have advantages over conventional components in having less non-physiologic fluid, improved logistics, and putative improvements in treating coagulopathy. LTOWB processing and implementation in resuscitation lacks standardization, and at the time of this writing, is not licensed or available in Canada.High quality evidence is emerging74 and retrospective evidence is promising.
Additional resources
Treat the Bleed
Developed by Canada’s leading experts in transfusion medicine, Treat the Bleed is a website that supports clinical decision making in the management of bleeding.
Podcast: The 7 Ts of Massive Hemorrhage Protocols
Helman, A. Callum, J. Haas, B. Petrosoniak, A. The 7 Ts of Massive Hemorrhage Protocols. Emergency Medicine Cases. February, 2021. https://emergencymedicinecases.com/7-ts-massive-hemorrhage-protocols/ Accessed 17 August 2021.
Continuing professional development credits
Fellows and health-care professionals who participate in the Canadian Royal College's Maintenance of Certification (MOC) program can claim the reading of the Clinical Guide to Transfusion as a continuing professional development (CPD) activity under Section 2: Individual learning. Learners can claim 0.5 credits per hour of reading to a maximum of 30 credits per year.
Medical laboratory technologists who participate in the Canadian Society for Medical Laboratory Science’s Professional Enhancement Program (PEP) can claim the reading of the Clinical Guide to Transfusion as a non-verified activity.
Suggested citation
Trudeau JD, Dawe P, Shih AW. Massive hemorrhage and emergency transfusion. In: Clarke G, Chargé S, editors. Clinical Guide to Transfusion [Internet]. Ottawa: Canadian Blood Services, 2021. [cited YYYY MM DD]. Chapter 11. Available at Professionaleducation.blood.ca
Acknowledgements
The authors acknowledge Jacqueline Trudeau, MD, PhD, FRCPC, as the author of the previous version of this chapter and thank Susan White, MLT, and Yulia Lin, MD, FRCPC, for their review of the current chapter.
If you have questions about the Clinical Guide to Transfusion or suggestions for improvement, please contact us through the Feedback form.
References
- Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A Multicenter, Randomized, Controlled Clinical Trial of Transfusion Requirements in Critical Care. New England Journal of Medicine 1999; 340: 409-17. https://www.nejm.org/doi/full/10.1056/NEJM199902113400601.
- Lacroix J, Hébert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, Gauvin F, Collet J-P, Toledano BJ, Robillard P, Joffe A, Biarent D, Meert K, Peters MJ. Transfusion Strategies for Patients in Pediatric Intensive Care Units. New England Journal of Medicine 2007; 356: 1609-19. https://www.nejm.org/doi/full/10.1056/NEJMoa066240.
- Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, Graupera I, Poca M, Alvarez-Urturi C, Gordillo J, Guarner-Argente C, Santaló M, Muñiz E, Guarner C. Transfusion Strategies for Acute Upper Gastrointestinal Bleeding. New England Journal of Medicine 2013; 368: 11-21. https://www.nejm.org/doi/full/10.1056/NEJMoa1211801.
- Robertson CS, Hannay HJ, Yamal J-M, Gopinath S, Goodman JC, Tilley BC, Investigators atESTT. Effect of Erythropoietin and Transfusion Threshold on Neurological Recovery after Traumatic Brain Injury: A Randomized Clinical Trial. JAMA 2014; 312: 36-47. https://doi.org/10.1001/jama.2014.6490.
- Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, Johansson PI, Åneman A, Vang ML, Winding R, Nebrich L, Nibro HL, Rasmussen BS, Lauridsen JRM, Nielsen JS, Oldner A, Pettilä V, Cronhjort MB, Andersen LH, Pedersen UG, Reiter N, Wiis J, White JO, Russell L, Thornberg KJ, Hjortrup PB, Müller RG, Møller MH, Steensen M, Tjäder I, Kilsand K, Odeberg-Wernerman S, Sjøbø B, Bundgaard H, Thyø MA, Lodahl D, Mærkedahl R, Albeck C, Illum D, Kruse M, Winkel P, Perner A. Lower Versus Higher Hemoglobin Threshold for Transfusion in Septic Shock. New England Journal of Medicine 2014; 371: 1381-91. https://www.nejm.org/doi/full/10.1056/NEJMoa1406617.
- Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, Nemo G, Dragert K, Beaupre L, Hildebrand K, Macaulay W, Lewis C, Cook DR, Dobbin G, Zakriya KJ, Apple FS, Horney RA, Magaziner J. Liberal or Restrictive Transfusion in High-Risk Patients after Hip Surgery. New England Journal of Medicine 2011; 365: 2453-62. https://www.nejm.org/doi/full/10.1056/NEJMoa1012452.
- Hajjar LA, Vincent J-L, Galas FRBG, Nakamura RE, Silva CMP, Santos MH, Fukushima J, Filho RK, Sierra DB, Lopes NH, Mauad T, Roquim AC, Sundin MR, Leão WC, Almeida JP, Pomerantzeff PM, Dallan LO, Jatene FB, Stolf NAG, Auler JOC. Transfusion Requirements after Cardiac Surgery: The Tracs Randomized Controlled Trial. JAMA 2010; 304: 1559-67. https://doi.org/10.1001/jama.2010.1446.
- Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, Reeves BC. Liberal or Restrictive Transfusion after Cardiac Surgery. New England Journal of Medicine 2015; 372: 997-1008. https://www.nejm.org/doi/full/10.1056/NEJMoa1403612.
- Mazer CD, Whitlock RP, Fergusson DA, Hall J, Belley-Cote E, Connolly K, Khanykin B, Gregory AJ, de Médicis É, McGuinness S, Royse A, Carrier FM, Young PJ, Villar JC, Grocott HP, Seeberger MD, Fremes S, Lellouche F, Syed S, Byrne K, Bagshaw SM, Hwang NC, Mehta C, Painter TW, Royse C, Verma S, Hare GMT, Cohen A, Thorpe KE, Jüni P, Shehata N. Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. New England Journal of Medicine 2017; 377: 2133-44. https://www.nejm.org/doi/full/10.1056/NEJMoa1711818.
- Etchill EW, Myers SP, McDaniel LM, Rosengart MR, Raval JS, Triulzi DJ, Peitzman AB, Sperry JL, Neal MD. Should All Massively Transfused Patients Be Treated Equally? An Analysis of Massive Transfusion Ratios in the Nontrauma Setting. Crit Care Med 2017; 45: 1311-6. https://journals.lww.com/ccmjournal/Fulltext/2017/08000/Should_All_Massively_Transfused_Patients_Be.7.aspx.
- Mesar T, Larentzakis A, Dzik W, Chang Y, Velmahos G, Yeh DD. Association between Ratio of Fresh Frozen Plasma to Red Blood Cells During Massive Transfusion and Survival among Patients without Traumatic Injury. JAMA Surgery 2017; 152: 574-80. https://doi.org/10.1001/jamasurg.2017.0098.
- Callum JL, Yeh CH, Petrosoniak A, McVey MJ, Cope S, Thompson T, Chin V, Karkouti K, Nathens AB, Murto K, Beno S, Pendergrast J, McDonald A, MacDonald R, Adhikari NKJ, Alam A, Arnold D, Barratt L, Beckett A, Brenneman S, Chaudhry HR, Collins A, Harvey M, Lampron J, Margarido C, McFarlan A, Nascimento B, Owens W, Pai M, Rizoli S, Ruijs T, Skeate R, Skelton T, Sholzberg M, Syer K, Viveiros J-L, Theriault J, Tinmouth A, Van Heest R, White S, Zeller M, Pavenski K. A Regional Massive Hemorrhage Protocol Developed through a Modified Delphi Technique. CMAJ Open 2019; 7: E546-E61. https://pubmed.ncbi.nlm.nih.gov/31484650
- Cotton BA, Au BK, Nunez TC, Gunter OL, Robertson AM, Young PP. Predefined Massive Transfusion Protocols Are Associated with a Reduction in Organ Failure and Postinjury Complications. J Trauma 2009; 66: 41-8; discussion 8-9. https://www.ncbi.nlm.nih.gov/pubmed/19131804.
- Cotton BA, Gunter OL, Isbell J, Au BK, Robertson AM, Morris JA, Jr., St Jacques P, Young PP. Damage Control Hematology: The Impact of a Trauma Exsanguination Protocol on Survival and Blood Product Utilization. J Trauma 2008; 64: 1177-82; discussion 82-3. https://www.ncbi.nlm.nih.gov/pubmed/18469638.
- O'Keeffe T, Refaai M, Tchorz K, Forestner JE, Sarode R. A Massive Transfusion Protocol to Decrease Blood Component Use and Costs. Arch Surg 2008; 143: 686-90; discussion 90-1. https://www.ncbi.nlm.nih.gov/pubmed/18645112.
- Mitra B, O'Reilly G, Cameron PA, Zatta A, Gruen RL. Effectiveness of Massive Transfusion Protocols on Mortality in Trauma: A Systematic Review and Meta-Analysis. ANZ Journal of Surgery 2013; 83: 918-23. https://onlinelibrary.wiley.com/doi/abs/10.1111/ans.12417.
- Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, Dubose JJ, Fox EE, Inaba K, Rodriguez CJ, Holcomb JB, Duchesne JC. Damage Control Resuscitation in Patients with Severe Traumatic Hemorrhage: A Practice Management Guideline from the Eastern Association for the Surgery of Trauma. Journal of Trauma and Acute Care Surgery 2017; 82: 605-17. https://journals.lww.com/jtrauma/Fulltext/2017/03000/Damage_control_resuscitation_in_patients_with.24.aspx.
- Meyer DE, Vincent LE, Fox EE, O'Keeffe T, Inaba K, Bulger E, Holcomb JB, Cotton BA. Every Minute Counts: Time to Delivery of Initial Massive Transfusion Cooler and Its Impact on Mortality. Journal of Trauma and Acute Care Surgery 2017; 83: 19-24. https://journals.lww.com/jtrauma/Fulltext/2017/07000/Every_minute_counts__Time_to_delivery_of_initial.4.aspx.
- Smith CE, Bauer AM, Pivalizza EG, Tanaka K, Boral L, Shander A, Waters JH. Massive Transfusion Protocol (Mtp) for Haemorrhagic Shock. ASA Committee on Blood Management, 2012: p. 1-11.
- Savage SA, Sumislawski JJ, Zarzaur BL, Dutton WP, Croce MA, Fabian TC. The New Metric to Define Large-Volume Hemorrhage: Results of a Prospective Study of the Critical Administration Threshold. Journal of Trauma and Acute Care Surgery 2015; 78: 224-30. https://journals.lww.com/jtrauma/Fulltext/2015/02000/The_new_metric_to_define_large_volume_hemorrhage_.2.aspx.
- Pommerening MJ, Goodman MD, Holcomb JB, Wade CE, Fox EE, Del Junco DJ, Brasel KJ, Bulger EM, Cohen MJ, Alarcon LH, Schreiber MA, Myers JG, Phelan HA, Muskat P, Rahbar M, Cotton BA, MPH on behalf of the PROMMTT Study Group. Clinical Gestalt and the Prediction of Massive Transfusion after Trauma. Injury 2015; 46: 807-13. https://pubmed.ncbi.nlm.nih.gov/25682314
- Shih AW, Al Khan S, Wang AY-H, Dawe P, Young PY, Greene A, Hudoba M, Vu E. Systematic Reviews of Scores and Predictors to Trigger Activation of Massive Transfusion Protocols. Journal of Trauma and Acute Care Surgery 2019; 87: 717-29. https://journals.lww.com/jtrauma/Fulltext/2019/09000/Systematic_reviews_of_scores_and_predictors_to.32.aspx.
- Mutschler M, Nienaber U, Münzberg M, Wölfl C, Schoechl H, Paffrath T, Bouillon B, Maegele M, The TraumaRegister D. The Shock Index Revisited – a Fast Guide to Transfusion Requirement? A Retrospective Analysis on 21,853 Patients Derived from the Traumaregister Dgu®. Crit Care 2013; 17: R172. https://doi.org/10.1186/cc12851.
- Spinella PC. Zero Preventable Deaths after Traumatic Injury: An Achievable Goal. Journal of Trauma and Acute Care Surgery 2017; 82: S2-S8. https://journals.lww.com/jtrauma/Fulltext/2017/06001/Zero_preventable_deaths_after_traumatic_injury__An.2.aspx.
- Brohi K, Singh J, Heron M, Coats T. Acute Traumatic Coagulopathy. J Trauma 2003; 54: 1127-30. https://www.ncbi.nlm.nih.gov/pubmed/12813333.
- Frith D, Brohi K. The Pathophysiology of Trauma-Induced Coagulopathy. Curr Opin Crit Care 2012; 18: 631-6. https://www.ncbi.nlm.nih.gov/pubmed/23010636.
- Curry NS, Davenport RA, Hunt BJ, Stanworth SJ. Transfusion Strategies for Traumatic Coagulopathy. Blood Rev 2012; 26: 223-32. https://www.ncbi.nlm.nih.gov/pubmed/22795890.
- Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, Cohen MJ, Cotton BA, Fabian TC, Inaba K, Kerby JD, Muskat P, O'Keeffe T, Rizoli S, Robinson BR, Scalea TM, Schreiber MA, Stein DM, Weinberg JA, Callum JL, Hess JR, Matijevic N, Miller CN, Pittet JF, Hoyt DB, Pearson GD, Leroux B, van Belle G, Group PS. Transfusion of Plasma, Platelets, and Red Blood Cells in a 1:1:1 Vs a 1:1:2 Ratio and Mortality in Patients with Severe Trauma: The Proppr Randomized Clinical Trial. JAMA 2015; 313: 471-82. https://www.ncbi.nlm.nih.gov/pubmed/25647203.
- Ley EJ, Clond MA, Srour MK, Barnajian M, Mirocha J, Margulies DR, Salim A. Emergency Department Crystalloid Resuscitation of 1.5 L or More Is Associated with Increased Mortality in Elderly and Nonelderly Trauma Patients. Journal of Trauma and Acute Care Surgery 2011; 70: 398-400. https://journals.lww.com/jtrauma/Fulltext/2011/02000/Emergency_Department_Crystalloid_Resuscitation_of.22.aspx.
- Jones DG, Nantais J, Rezende-Neto JB, Yazdani S, Vegas P, Rizoli S. Crystalloid Resuscitation in Trauma Patients: Deleterious Effect of 5l or More in the First 24h. BMC Surgery 2018; 18: 93. https://doi.org/10.1186/s12893-018-0427-y.
- Henry S. Atls 10th Edition Offers New Insights into Managing Trauma Patients Bulletin of the American College of Surgeons. American College of Surgeons, 2018. https://bulletin.facs.org/2018/06/atls-10th-edition-offers-new-insights-into-managing-trauma-patients/#Chapter_3_Shock.
- Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, Komadina R, Maegele M, Nardi G, Riddez L, Samama C-M, Vincent J-L, Rossaint R. The European Guideline on Management of Major Bleeding and Coagulopathy Following Trauma: Fifth Edition. Crit Care 2019; 23: 98. https://doi.org/10.1186/s13054-019-2347-3.
- Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The Ratio of Blood Products Transfused Affects Mortality in Patients Receiving Massive Transfusions at a Combat Support Hospital. J Trauma 2007; 63: 805-13. https://www.ncbi.nlm.nih.gov/pubmed/18090009.
- Etchill EW, Myers SP, McDaniel LM, Rosengart MR, Raval JS, Triulzi DJ, Peitzman AB, Sperry JL, Neal MD. Should All Massively Transfused Patients Be Treated Equally? An Analysis of Massive Transfusion Ratios in the Nontrauma Setting. Crit Care Med 2017. https://www.ncbi.nlm.nih.gov/pubmed/28537938.
- Mesar T, Larentzakis A, Dzik W, Chang Y, Velmahos G, Yeh DD. Association between Ratio of Fresh Frozen Plasma to Red Blood Cells During Massive Transfusion and Survival among Patients without Traumatic Injury. JAMA Surg 2017; 152: 574-80. https://www.ncbi.nlm.nih.gov/pubmed/28273299.
- CRASH-2 collaborators, Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, Morales C, Perel P, Prieto-Merino D, Woolley T. The Importance of Early Treatment with Tranexamic Acid in Bleeding Trauma Patients: An Exploratory Analysis of the Crash-2 Randomised Controlled Trial. Lancet 2011; 377: 1096-101, 101 e1-2. https://www.ncbi.nlm.nih.gov/pubmed/21439633.
- Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (Matters) Study. Archives of Surgery 2012; 147: 113-9. https://doi.org/10.1001/archsurg.2011.287.
- WOMAN Trial Collaborators. Effect of Early Tranexamic Acid Administration on Mortality, Hysterectomy, and Other Morbidities in Women with Post-Partum Haemorrhage (Woman): An International, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2017; 389: 2105-16. https://www.ncbi.nlm.nih.gov/pubmed/28456509.
- Myles PS, Smith JA, Forbes A, Silbert B, Jayarajah M, Painter T, Cooper DJ, Marasco S, McNeil J, Bussières JS, McGuinness S, Byrne K, Chan MTV, Landoni G, Wallace S. Tranexamic Acid in Patients Undergoing Coronary-Artery Surgery. New England Journal of Medicine 2016; 376: 136-48. https://www.nejm.org/doi/full/10.1056/NEJMoa1606424.
- Fillingham YA, Ramkumar DB, Jevsevar DS, Yates AJ, Bini SA, Clarke HD, Schemitsch E, Johnson RL, Memtsoudis SG, Sayeed SA, Sah AP, Della Valle CJ. Tranexamic Acid Use in Total Joint Arthroplasty: The Clinical Practice Guidelines Endorsed by the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. J Arthroplasty 2018; 33: 3065-9. https://pubmed.ncbi.nlm.nih.gov/30146350/.
- Lu VM, Ho Y-T, Nambiar M, Mobbs RJ, Phan K. The Perioperative Efficacy and Safety of Antifibrinolytics in Adult Spinal Fusion Surgery: A Systematic Review and Meta-Analysis. Spine 2018; 43: E949-E58. https://journals.lww.com/spinejournal/Fulltext/2018/08150/The_Perioperative_Efficacy_and_Safety_of.16.aspx.
- Crash-3 trial collaborators. Effects of Tranexamic Acid on Death, Disability, Vascular Occlusive Events and Other Morbidities in Patients with Acute Traumatic Brain Injury (Crash-3): A Randomised, Placebo-Controlled Trial. Lancet (London, England) 2019; 394: 1713-23. https://pubmed.ncbi.nlm.nih.gov/31623894
- McQuilten ZK WE, Bailey M, Cameron PA, Cooper DJ. Fibrinogen Is an Independent Predictor of Mortality in Major Trauma Patients: A Five-Year Statewide Cohort Study. Injury 2017; May;48(5): 1074-81. https://pubmed.ncbi.nlm.nih.gov/28190583/.
- Curry NS, Davenport RA, Hunt BJ, Stanworth SJ. Transfusion Strategies for Traumatic Coagulopathy. Blood Rev 2012; 26: 223-32. http://www.sciencedirect.com/science/article/pii/S0268960X12000483.
- Karkouti K, Callum J, Crowther MA, McCluskey SA, Pendergrast J, Tait G, Yau TM, Beattie WS. The Relationship between Fibrinogen Levels after Cardiopulmonary Bypass and Large Volume Red Cell Transfusion in Cardiac Surgery: An Observational Study. Anesth Analg 2013; 117: 14-22. https://www.ncbi.nlm.nih.gov/pubmed/23687229.
- Charbit B, Mandelbrot L, Samain E, Baron G, Haddaoui B, Keita H, Sibony O, Mahieu-Caputo D, Hurtaud-Roux MF, Huisse MG, Denninger MH, de Prost D, Group PPHS. The Decrease of Fibrinogen Is an Early Predictor of the Severity of Postpartum Hemorrhage. J Thromb Haemost 2007; 5: 266-73. https://www.ncbi.nlm.nih.gov/pubmed/17087729.
- Kozek-Langenecker SA, Ahmed AB, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, De Robertis E, Faraoni D, Filipescu DC, Fries D, Haas T, Jacob M, Lance MD, Pitarch JVL, Mallett S, Meier J, Molnar ZL, Rahe-Meyer N, Samama CM, Stensballe J, Van der Linden PJF, Wikkelso AJ, Wouters P, Wyffels P, Zacharowski K. Management of Severe Perioperative Bleeding: Guidelines from the European Society of Anaesthesiology: First Update 2016. Eur J Anaesthesiol 2017; 34: 332-95. https://www.ncbi.nlm.nih.gov/pubmed/28459785.
- Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Hunt BJ, Komadina R, Nardi G, Neugebauer E, Ozier Y, Riddez L, Schultz A, Stahel PF, Vincent JL, Spahn DR, Task Force for Advanced Bleeding Care in T. Management of Bleeding Following Major Trauma: An Updated European Guideline. Crit Care 2010; 14: R52. https://www.ncbi.nlm.nih.gov/pubmed/20370902.
- Baird EJ. Identification and Management of Obstetric Hemorrhage. Anesthesiol Clin 2017; 35: 15-34. https://www.ncbi.nlm.nih.gov/pubmed/28131117.
- Itagaki Y, Hayakawa M, Maekawa K, Saito T, Kodate A, Honma Y, Mizugaki A, Yoshida T, Ohyasu T, Katabami K, Wada T. Early Administration of Fibrinogen Concentrate Is Associated with Improved Survival among Severe Trauma Patients: A Single-Centre Propensity Score-Matched Analysis. World Journal of Emergency Surgery 2020; 15: 7. https://doi.org/10.1186/s13017-020-0291-9.
- Innerhofer P, Fries D, Mittermayr M, Innerhofer N, von Langen D, Hell T, Gruber G, Schmid S, Friesenecker B, Lorenz IH, Ströhle M, Rastner V, Trübsbach S, Raab H, Treml B, Wally D, Treichl B, Mayr A, Kranewitter C, Oswald E. Reversal of Trauma-Induced Coagulopathy Using First-Line Coagulation Factor Concentrates or Fresh Frozen Plasma (Retic): A Single-Centre, Parallel-Group, Open-Label, Randomised Trial. The Lancet Haematology 2017; 4: e258-e71. https://doi.org/10.1016/S2352-3026(17)30077-7.
- Nascimento B CJ, Tien H, Peng H, Rizoli S, Karanicolas P, Alam A, Xiong W, Selby R, Garzon AM, Colavecchia C, Howald R, Nathens A, Beckett A. Fibrinogen in the Initial Resuscitation of Severe Trauma (Fiirst): A Randomized Feasibility Trial. Br J Anaesth 2016; Dec;117(6): 775-82. https://pubmed.ncbi.nlm.nih.gov/27956676/.
- Winearls J, Wullschleger M, Wake E, Hurn C, Furyk J, Ryan G, Trout M, Walsham J, Holley A, Cohen J, Shuttleworth M, Dyer W, Keijzers G, Fraser JF, Presneill J, Campbell D. Fibrinogen Early in Severe Trauma Study (Feisty): Study Protocol for a Randomised Controlled Trial. Trials 2017; 18: 241. https://doi.org/10.1186/s13063-017-1980-x.
- ClinicalTrials.gov. Factor in the Initial Resuscitation of Severe Trauma 2 Patients (Fiirst-2), Identifier: Nct04534751. Bethesda (MD), U.S. National Library of Medicine 2020. https://clinicaltrials.gov/ct2/show/NCT04534751 (Last accessed June 1 2021).
- Clinical Trials Unit. Cryostat-2: Early Cryoprecipitate in Trauma. Cambridge (UK), NHS Blood and Transplant 2021. https://www.nhsbt.nhs.uk/clinical-trials-unit/current-trials-and-studies/cryostat-2/ (Last accessed June 1 2021).
- Callum J, Farkouh ME, Scales DC, Heddle NM, Crowther M, Rao V, Hucke H-P, Carroll J, Grewal D, Brar S, Bussières J, Grocott H, Harle C, Pavenski K, Rochon A, Saha T, Shepherd L, Syed S, Tran D, Wong D, Zeller M, Karkouti K, Group ftFR. Effect of Fibrinogen Concentrate Vs Cryoprecipitate on Blood Component Transfusion after Cardiac Surgery: The Fibres Randomized Clinical Trial. JAMA 2019; 322: 1966-76. https://doi.org/10.1001/jama.2019.17312.
- Ontario Regional Blood Coordinating Network. Preserving the Supply of Type O Rh(D) Negative Red Blood Cells by Re-Defining the Maximum Age at Which Ontario Women Are Considered to Be of Child-Bearing Potential. transfusionontario.org, ORBCoN, 2018. https://transfusionontario.org/wp-content/uploads/2020/06/Age-of-Child-Bearing-Potential-for-Ontario-Women-in-2018.pdf.
- Fung Kee Fung K, Eason E, Crane J, Armson A, De La Ronde S, Farine D, Keenan-Lindsay L, Leduc L, Reid GJ, Aerde JV, Wilson RD, Davies G, Desilets VA, Summers A, Wyatt P, Young DC, Maternal-Fetal Medicine Committee GC. Prevention of Rh Alloimmunization. J Obstet Gynaecol Can 2003; 25: 765-73. https://www.ncbi.nlm.nih.gov/pubmed/12970812.
- Canadian Standards Association Group. Can/Csa-Z902:20 - Blood and Blood Components. Published in Canada by CSA, 2020.
- Flommersfeld S, Mand C, Kühne CA, Bein G, Ruchholtz S, Sachs UJ. Unmatched Type O Rhd+ Red Blood Cells in Multiple Injured Patients. Transfusion Medicine and Hemotherapy 2018; 45: 158-61. https://www.karger.com/DOI/10.1159/000485388.
- Yazer M, Triulzi D, Sperry J, Corcos A, Seheult J. Rate of Rhd-Alloimmunization after the Transfusion of Rhd-Positive Red Blood Cell Containing Products among Injured Patients of Childbearing Age: Single Center Experience and Narrative Literature Review. Hematology 2021; 26: 321-7. https://doi.org/10.1080/16078454.2021.1905395.
- O'Brien KL, Kim YA, Haspel RL, Uhl L. Provision of Kel1-Negative Blood to Obstetric Patients: A 3-Year Single-Institution Retrospective Review. Transfusion 2015; 55: 599-604; quiz 598. http://www.ncbi.nlm.nih.gov/pubmed/25118004.
- Saverimuttu J, Greenfield T, Rotenko I, Crozier J, Jalaludin B, Harvey M. Implications for Urgent Transfusion of Uncrossmatched Blood in the Emergency Department: The Prevalence of Clinically Significant Red Cell Antibodies within Different Patient Groups. Emerg Med (Fremantle) 2003; 15: 239-43. https://www.ncbi.nlm.nih.gov/pubmed/12786645.
- Dutton RP, Shih D, Edelman BB, Hess J, Scalea TM. Safety of Uncrossmatched Type-O Red Cells for Resuscitation from Hemorrhagic Shock. J Trauma 2005; 59: 1445-9. https://www.ncbi.nlm.nih.gov/pubmed/16394920.
- Mulay SB, Jaben EA, Johnson P, Badjie K, Stubbs JR. Risks and Adverse Outcomes Associated with Emergency-Release Red Blood Cell Transfusion. Transfusion 2013; 53: 1416-20. https://www.ncbi.nlm.nih.gov/pubmed/23067326.
- Yazer MH, Spinella PC, Allard S, Roxby D, So-Osman C, Lozano M, Gunn K, Shih AW, Stensballe J, Johansson PI, Bagge Hansen M, Maegele M, Doughty H, Crombie N, Jenkins DH, McGinity AC, Schaefer RM, Martinaud C, Shinar E, Strugo R, Chen J, Russcher H. Vox Sanguinis International Forum on the Use of Prehospital Blood Products and Pharmaceuticals in the Treatment of Patients with Traumatic Hemorrhage. Vox Sang 2018; 113: 816-30. https://onlinelibrary.wiley.com/doi/abs/10.1111/vox.12677.
- Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, Adams PW, Daley BJ, Miller RS, Harbrecht BG, Claridge JA, Phelan HA, Witham WR, Putnam AT, Duane TM, Alarcon LH, Callaway CW, Zuckerbraun BS, Neal MD, Rosengart MR, Forsythe RM, Billiar TR, Yealy DM, Peitzman AB, Zenati MS. Prehospital Plasma During Air Medical Transport in Trauma Patients at Risk for Hemorrhagic Shock. New England Journal of Medicine 2018; 379: 315-26. https://www.nejm.org/doi/full/10.1056/NEJMoa1802345.
- Moore HB, Moore EE, Chapman MP, McVaney K, Bryskiewicz G, Blechar R, Chin T, Burlew CC, Pieracci F, West FB, Fleming CD, Ghasabyan A, Chandler J, Silliman CC, Banerjee A, Sauaia A. Plasma-First Resuscitation to Treat Haemorrhagic Shock During Emergency Ground Transportation in an Urban Area: A Randomised Trial. The Lancet 2018; 392: 283-91. https://doi.org/10.1016/S0140-6736(18)31553-8.
- Pusateri AE, Moore EE, Moore HB, Le TD, Guyette FX, Chapman MP, Sauaia A, Ghasabyan A, Chandler J, McVaney K, Brown JB, Daley BJ, Miller RS, Harbrecht BG, Claridge JA, Phelan HA, Witham WR, Putnam AT, Sperry JL. Association of Prehospital Plasma Transfusion with Survival in Trauma Patients with Hemorrhagic Shock When Transport Times Are Longer Than 20 Minutes: A Post Hoc Analysis of the Pamper and Combat Clinical Trials. JAMA Surgery 2020; 155: e195085-e. https://doi.org/10.1001/jamasurg.2019.5085.
- Guyette FX, Sperry JL, Peitzman AB, Billiar TR, Daley BJ, Miller RS, Harbrecht BG, Claridge JA, Putnam T, Duane TM, Phelan HA, Brown JB. Prehospital Blood Product and Crystalloid Resuscitation in the Severely Injured Patient: A Secondary Analysis of the Prehospital Air Medical Plasma Trial. Ann Surg 2021; 273: 358-64. https://journals.lww.com/annalsofsurgery/Fulltext/2021/02000/Prehospital_Blood_Product_and_Crystalloid.23.aspx.
- Reitz KM, Moore HB, Guyette FX, Sauaia A, Pusateri AE, Moore EE, Hassoune A, Chapman MP, Daley BJ, Miller RS, Harbrecht BG, Claridge JA, Phelan HA, Brown JB, Zuckerbraun BS, Neal MD, Yazer MH, Sperry JL. Prehospital Plasma in Injured Patients Is Associated with Survival Principally in Blunt Injury: Results from Two Randomized Prehospital Plasma Trials. Journal of Trauma and Acute Care Surgery 2020; 88: 33-41. https://journals.lww.com/jtrauma/Fulltext/2020/01000/Prehospital_plasma_in_injured_patients_is.5.aspx.
- Dunbar NM, Yazer MH, Collaborative obotBEfST, Investigators tSS. Safety of the Use of Group a Plasma in Trauma: The Stat Study. Transfusion 2017; 57: 1879-84. https://onlinelibrary.wiley.com/doi/abs/10.1111/trf.14139.
- Seheult JN, Dunbar NM, Hess JR, Tuott EE, Bahmanyar M, Campbell J, Fontaine M, Khan J, Ko A, Mi J, Murphy MF, Nykoluk T, Poisson J, Raval JS, Shih A, Sperry JL, Staves J, Wong M, Yan MTS, Ziman A, Yazer MH, collaborative TBEfST. Transfusion of Blood Components Containing Abo-Incompatible Plasma Does Not Lead to Higher Mortality in Civilian Trauma Patients. Transfusion 2020; 60: 2517-28. https://onlinelibrary.wiley.com/doi/abs/10.1111/trf.16008.
- Leeper CM, Yazer MH, Neal MD. Whole-Blood Resuscitation of Injured Patients’ Plasma. JAMA Surgery 2021; 156: 101-2. https://doi.org/10.1001/jamasurg.2020.4116.