Ce système de groupes sanguins découvert par Landsteiner et Levin en 1927 a été nommé d’après les trois premiers antigènes identifiés dans le système : M, N et S. À l’heure actuelle, on compte 50 antigènes dans ce système.2 Les gènes GYPA et GYPB codent pour la glycophorine A et la glycophorine B, des produits qui agissent comme chaperons pour le transport de la bande 3 jusqu’à la membrane des globules rouges. Les antigènes M et N sont portés par la glycophorine A et les antigènes S et s par la glycophorine B.3, 4
Anti-M
À retenir
- L’anti-M a parfois une importance clinique.1 Les patients chez lesquels l’anti-M est détectable à 37 °C (lors du test indirect à l’antiglobuline) peuvent se faire transfuser des unités de CGR compatible par épreuve croisée à l’antiglobuline à 37 °C.
- Les personnes drépanocytaires porteuses de l’anti-M doivent se faire transfuser des unités de CGR dépourvu de l’antigène M.5, 6
- Si une femme enceinte possède un anti-M de type IgG qui réagit à 37 °C, elle doit se prêter à un titrage continu et il est préconisé que le père de l’enfant fasse l’objet d’un test de dépistage de l’antigène M.7
Introduction
L’antigène M fait partie du système sanguin MNS et se trouve à la surface des globules rouges, sur une glycoprotéine appelée glycophorine A.2 L’antigène M est présent chez 74 % à 78 % de la plupart des populations.
L’anticorps anti-M peut être naturellement présent dans l’organisme (c’est-à-dire sans qu’il y ait eu d’exposition aux globules rouges lors d’une transfusion ou pendant une grossesse) ou être le produit d’une réaction immunitaire. Dans les deux cas, il s’agit majoritairement d’un anticorps de type IgM avec un composant IgG, souvent associé à d’autres anticorps.3 Les anticorps anti-M ont souvent une faible amplitude thermique et une réactivité prédominante à basse température. Si un « nouvel » anti-M est détecté au stade prénatal, il n’est pas rare de constater à l’accouchement que le bébé est dépourvu de l’antigène M. Cela constitue une preuve par inférence que l’anti-M détecté au stade prénatal dans le plasma maternel est naturel, et non le produit d’une réaction immunitaire.
En médecine transfusionnelle, l’anti-M est cliniquement négligeable pour la plupart des patients.8 Il n’a généralement aucun lien avec les réactions transfusionnelles hémolytiques aiguës ou retardées, sauf pour certaines personnes drépanocytaires chez qui il peut entraîner une hémolyse, voire précipiter une hyperhémolyse.5
L’anti-M est rarement associé à la maladie hémolytique du nouveau-né ou du fœtus (MHNNF).1, 9
Le typage de l’antigène M ne fait pas partie des phénotypages systématiques réalisés par la Société canadienne du sang sur ses plateformes automatisées. Par conséquent, les demandes d’unités de CGR dépourvu de l’antigène M nécessitent un phénotypage ou un génotypage manuel à part.
Prise en charge : Épreuves prétransfusionnelles et prénatales
Épreuves prétransfusionnelles
Lorsqu’on détecte l’anti-M avant une transfusion, il n’a généralement pas d’importance clinique. Il n’est pas nécessaire de sélectionner des unités de CGR dépourvu de l’antigène M. On peut, à la place, transfuser des unités de CGR compatibilisé par test indirect à l’antiglobuline à 37 °C.1 Certaines recommandations transfusionnelles internationales suggèrent de sélectionner des unités de CGR dépourvu de l’antigène M si l’anticorps est réactif au test indirect à l’antiglobuline, mais cette pratique n’est pas uniforme au Canada.
Dans les tests indirects à l’antiglobuline, l’anti-M peut réagir plus fortement sur gel qu’en tube, ce qui peut entraîner une incompatibilité lors de l’épreuve croisée sur gel. Si cela se produit, il est possible d’avoir recours à un test indirect à l’antiglobuline en tube utilisant une solution saline pour trouver des unités compatibles. De plus, un préchauffage peut parfois s’avérer utile.10 L’anti-M est souvent plus réactif à température ambiante ou à 4 °C. L’anti-M est souvent plus réactif à température ambiante ou à 4 °C, même s’il s’agit d’un anticorps de type IgG. Le préchauffage peut donc diminuer ou éliminer la réactivité de l’anti-M, ce qui permet de réaliser l’épreuve de compatibilité.
L’antigène M n’est généralement pas pris en compte lors de la recherche de globules rouges phénotypiquement compatibles. Pour les personnes qui suivent un traitement pouvant induire une hypothermie, y compris certaines interventions chirurgicales cardiaques, certains services de transfusion recommandent de transfuser des unités dépourvues de l’antigène M aux patients porteurs de l’anti-M. Aucune preuve ne vient étayer cette exigence et certaines données semblent indiquer que cette pratique n’est pas nécessaire.11
Consulter le tableau récapitulatif des recommandations transfusionnelles pour les anticorps non ABO pour une synthèse des recommandations relatives à la transfusion de globules rouges chez les patients présentant des anticorps non ABO.
Épreuves prénatales
L’anti-M est un anticorps couramment détecté dans les échantillons prénataux. La plupart du temps, il n’a pas d’importance clinique, car il s’agit majoritairement d’un anticorps de type IgM qui ne traverse pas la barrière placentaire.
Pour distinguer les anti-M de type IgM des anti-M de type IgG, il existe certaines méthodes d’identification des anticorps qui excluent les anticorps IgM (figure 1). De plus, des techniques spéciales telles que le traitement du plasma par dithiothréitol (DTT) peuvent aider à les distinguer. Lorsque l’anti-M est un anticorps de type IgG qui réagit à 37 °C et qu’il présente un titre élevé ou en augmentation, il peut s’agir d’un anticorps cliniquement significatif susceptible de traverser le placenta et d’affecter le fœtus. Dans ces cas, le père de l’enfant doit faire l’objet d’un test de dépistage de l’antigène M. Les taux d’anti-M de la mère sont régulièrement titrés pendant la période prénatale. On préconise une méthode de surveillance des anti-M qui consiste à effectuer des titrages intermittents pour détecter les titres en augmentation, avec un suivi régulier uniquement dans les rares cas où les taux d’anticorps augmentent.12, 13
Il est très rare que l’anti-M atteigne un titre critique nécessitant une consultation en médecine materno-fœtale pour une surveillance fœtale plus approfondie. L’anti-M peut agir par suppression de l’érythropoïèse fœtale plutôt que par hémolyse, ce qui peut entraîner une anémie néonatale en plus ou à la place de l’anémie fœtale. On a déjà signalé quelques cas d’anémie fœtale intra-utérine sévère due aux anti-M.7, 9 Cela reste toutefois très exceptionnel et, malgré la présence relativement courante de l’anti-M chez les femmes enceintes, il est très rare de constater une anémie fœtale ou néonatale significative en Amérique du Nord.
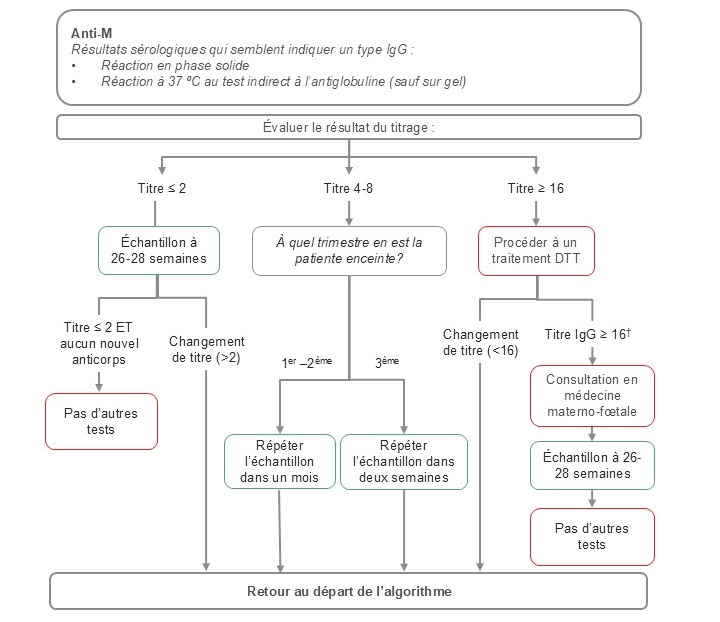
Figure 1. Algorithme suggéré pour le dépistage prénatal des anticorps anti-M.
Note : Il s’agit d’un algorithme adapté et son utilisation doit se conformer aux conditions de votre laboratoire et à votre patientèle. Cet algorithme ne doit pas être utilisé pour les patients ayant des antécédents de MHNNF due à des anti-M. Dans ce cas, une orientation précoce vers un spécialiste en médecine materno-fœtale est indiquée. Sauf indication contraire, le titrage a été effectué sur du plasma pur.
†Plasma traité par DTT = titre IgG
Patients drépanocytaires
Pour les patients drépanocytaires porteurs d’un anticorps anti-M, il convient de transfuser des unités de CGR dépourvu de l’antigène M, en plus de réaliser un phénotypage du profil d’antigènes et d’anticorps des globules rouges du patient. 5, 14 Dans ce contexte clinique, l’anti-M peut être associé à des réactions hémolytiques; il est donc recommandé de garantir la compatibilité en transfusant des unités de CGR dépourvu de l’antigène M.
Glycophorines hybrides (anti-Mi(a), anti-Mur, anti-Bun, anti-Vw, anti-HUT)
À retenir
- Mi(a) désigne un groupe d’antigènes à faible prévalence formés à la suite de réarrangements hybrides des gènes codant pour les antigènes MN et Ss (glycophorine A et B respectivement). C’est notamment le cas de GP.Mur, GP.Hut et GP.Vw.
- Les antigènes Mi(a) peuvent être présents chez 6 à 15 % des personnes d’origine asiatique, mais sont relativement rares chez les personnes d’origine européenne (0,1 à 0,2 %).
- Les anticorps anti-Mi(a) et les anticorps apparentés peuvent provoquer une maladie hémolytique du nouveau-né ou du fœtus (MHNNF), qui peut être sans gravité, mais qui peut aussi s’avérer mortelle.
- À l’instar des autres anticorps dirigés contre les antigènes à faible prévalence, les anticorps anti-Mi(a) ne sont généralement pas détectés lors d’un dépistage prénatal, car les cellules sont dépourvues de l’antigène Mi(a). Les anticorps dirigés contre les antigènes à faible prévalence sont souvent détectés lorsque le nouveau-né présente un ictère et/ou un TDA positif, et que le dépistage des anticorps maternels est négatif.
- Les anticorps anti-Mi(a) sont rarement associés à des réactions transfusionnelles hémolytiques.
Introduction
Les glycophorines hybrides sont un groupe d’antigènes présents sur la glycophorine A (GPA) et la glycophorine B (GPB). Ils résultent de fusions et de réarrangements génétiques complexes. Contrairement aux polymorphismes nucléotidiques, qui produisent plusieurs des variantes d’antigènes des groupes sanguins courants, ces réarrangements peuvent entraîner l’expression de chaînes d’acides aminés comportant plusieurs nouveaux antigènes, qui peuvent à leur tour stimuler plusieurs anticorps discrets (figure 2).
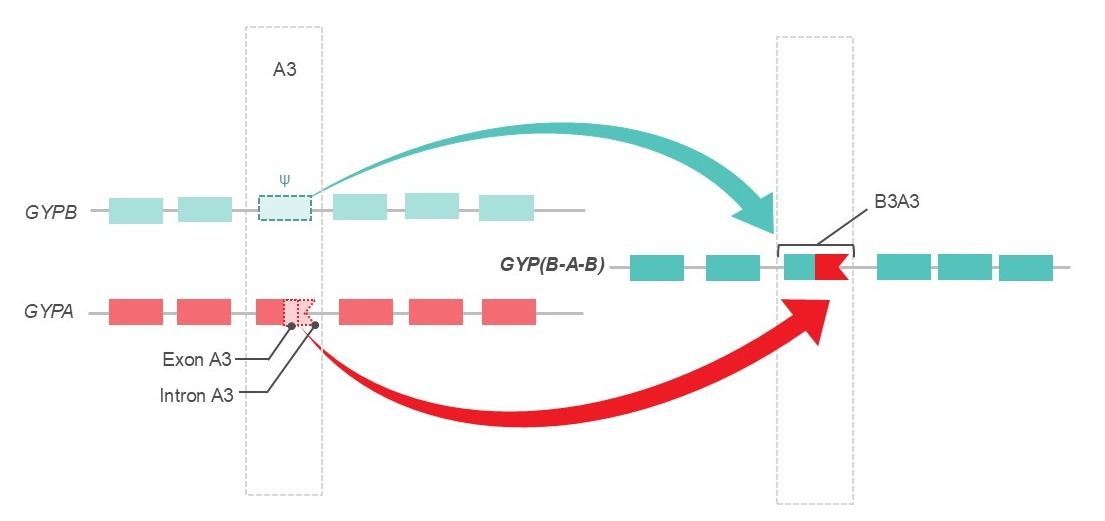
Figure 2. Exemple d’un épisode de recombinaison génétique observé dans les glycophorines hybrides GP.Mur, GP.Bun, GP.Hop et GP.HF. Dans ces hybrides, une partie de la glycophorine A (GYPA) remplace l’extrémité du pseudoexon B3 (Ψ) de la glycophorine B (GYPB). Cela ajoute un site d’épissage fonctionnel à B3, qui s’exprime alors anormalement, ce qui conduit à l’expression de plusieurs nouveaux antigènes : Mi(a), Mur, Hil, MUT et MINY 2,3. Figure adaptée de Daniels et Sanger, 2013.3
Mi(a) tire son nom de la série Miltenberger, une ancienne convention d’appellation. C’est la convention d’appellation Reid et Tippett qui la remplace désormais15, 16, car elle intègre des données de séquençage et biochimiques qui permettent de mieux caractériser ces phénotypes (tableau 1). Il est important de noter que le nom « Mi(a) » est généralement utilisé pour désigner un groupe d’antigènes qui comprend notamment Mur et Vw. Cependant, on a également découvert que Mi(a) est un antigène discret avec un anticorps monoclonal offert sur le marché2, ce qui ajoute à la confusion dans la nomenclature. Ainsi, si un patient a développé un nouvel anti-Mi(a), il se peut que cet anticorps réagisse à un seul antigène (généralement Mur ou Vw), mais il se peut aussi que plusieurs anticorps réagissent avec l’un des phénotypes contenant Mi(a) (tableau 1).
Tableau 1. Définition sérologique des phénotypes de Miltenberger et notation de remplacement, adaptée de Daniels et Sanger, 2013 (p. 118)2–4.
| Phénotypes | Antigènes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Famille Mi | Nouvelle notation (Reid et Tippett) | Mi(a) | Vw | Mur | Hil | Hut | MUT | Hop | Nob | DANE | TSEN | MINY |
| Mi.I | GP.Vw | + | + | - | - | - | - | - | - | - | - | - |
| Mi.II | GP.Hut | + | - | - | - | + | + | - | - | - | - | - |
| Mi.III | GP.Mur | + | - | + | + | - | + | - | - | - | - | + |
| Mi.IV | GP.Hop | + | - | + | - | - | + | + | - | - | + | + |
| Mi.V | GP.Hil | - | - | - | + | - | - | - | - | - | - | + |
| Mi.VI | GP.Bun | + | - | + | + | - | + | + | - | - | - | + |
| Mi.VII | GP.Nob | - | - | - | - | - | - | - | + | - | - | - |
| Mi.VIII | GP.Joh | - | - | - | - | - | - | + | + | - | NT | - |
| Mi.IX | GP.Dane | - | - | + | - | - | - | - | - | + | - | - |
| Mi.X | GP.HF | + | - | - | + | - | + | - | - | - | - | + |
| Mi.XI | GP.JL | - | - | - | - | - | NT | - | - | - | + | + |
License number: 6110920764012
NT: non testé
GP.Mur est le phénotype le plus courant.17 L’incidence de cette glycophorine hybride chez les personnes d’origine caucasienne est faible et estimée à 0,0098 %; cependant, elle est beaucoup plus élevée chez les personnes originaires de Chine et d’Asie du Sud-Est (6 à 15 %).2, 3 Il existe des variations importantes au sein de ce grand groupe ethnique, l’incidence atteignant 88 % chez certaines sous-populations.18 GP.Bun n’est pas toujours signalé comme une entité distincte de GP.Mur. Dans une étude menée sur des personnes d’origine thaïlandaise, 9,03 % étaient porteuses de Mi(a) (selon la sérologie), parmi lesquelles 88,3 % étaient porteuses de GP.Mur et 11,7 % de GP.Bun selon le séquençage génétique; aucune autre glycophorine hybride n’était détectée.19 D’autres glycophorines hybrides ont été signalées dans des populations d’ascendance européenne à des fréquences proches de 0,05 %, GP.Vw atteignant jusqu'à 1,4 % chez les personnes d’ascendance suisse.17 Heathcote et coll. 17et Mallari et coll.20 ont rédigé des revues de cas exhaustives qui incluent des résumés plus détaillés de l’épidémiologie de ces phénotypes. Parmi les déclarations de réaction transfusionnelle hémolytique ou MHNNF sévère, 94 % font suite à une exposition à GP.Mur et GP.Vw.17
Le typage de l’antigène Mi(a) ne fait pas partie des phénotypages systématiques réalisés par la Société canadienne du sang sur ses plateformes automatisées. Par conséquent, les demandes d’unités de CGR dépourvu de l’antigène Mi(a) nécessitent un phénotypage ou un génotypage manuel à part.
Prise en charge : Épreuves prétransfusionnelles et prénatales
ÉPREUVES PRÉTRANSFUSIONNELLES
La détection de l’anti-Mi(a) chez les patientes non enceintes ne pose généralement pas de problème majeur pour l’approvisionnement en sang dans les pays occidentaux, car la plupart des donneurs sont dépourvus de l’antigène Mi(a).
Les anticorps dirigés contre les glycophorines hybrides peuvent être naturellement présents dans l’organisme ou être le produit d’une réaction immunitaire. Les anticorps naturellement présents dans l’organisme sont généralement de type IgM et n’ont pas d’importance clinique.17 Ce phénomène est bien connu pour les anticorps anti-Vw, présents chez 0,5 % des personnes sans antécédents de grossesse ou de transfusion,2 et c’est la raison pour laquelle les globules rouges porteurs de Vw ne sont intentionnellement pas inclus dans les panels de dépistage.17 Heathcote et coll.17 fournissent une revue complète de la littérature sur les réactions transfusionnelles hémolytiques. Ils décrivent un cas mortel de réaction transfusionnelle hémolytique; le patient avait réagi à une unité de CGR porteur de GP.Vw.21 Tous les cas de réaction transfusionnelle hémolytique présentaient des IgG titrant de 8 à 512. Il n’y a eu aucun cas déclaré de réaction transfusionnelle hémolytique avec uniquement des IgM. Les anticorps les plus fréquemment impliqués dans ce groupe sont les anti-Mi(a), les anti-Mur, les anti-MUT et, dans une moindre mesure, les anti-Hil.
En cas de suspicion d’anticorps dirigés contre les antigènes à faible prévalence chez des patientes non enceintes, les échantillons peuvent être envoyés à un laboratoire de référence pour des épreuves sérologiques, et un test génétique peut être envisagé si une confirmation est nécessaire.22, 23 Bien que les anti-Mi(a) puissent provoquer une réaction transfusionnelle hémolytique sévère,17 il est peu probable que ces patientes soient exposées à l’antigène Mi(a) dans des pays comme le Canada, où sa prévalence est faible dans le bassin de donneurs. Comme pour les autres anticorps présentant un résultat positif, les patientes peuvent se faire transfuser en toute sécurité des unités compatibles par épreuve croisée à l’antiglobuline. Des tests de classe d’anticorps par traitement du plasma par dithiothréitol peuvent être envisagés pour déterminer la présence d’IgG et/ou d’IgM, car les anticorps de type IgM seulement n’ont pas d’importance clinique.17, 23 Il n’est pas nécessaire de se procurer des unités dépourvues de Mi(a), et le dépistage de l’antigène Mi(a) chez les donneurs n’est pas systématique à la Société canadienne du sang. Les antisérums sont difficiles à trouver et les demandes de tests peuvent entraîner des retards inutiles dans la transfusion.
ÉPREUVES PRÉNATALES
Bien que rarement déclaré, l’anti-Mi(a) présente un risque de MHNNF légère à mortelle.2, 3, 17, 20, 24 Il existe des preuves évidentes que les anticorps IgG à titre élevé dirigés contre les glycophorines hybrides constituent un risque important de MHNNF, tandis que les anticorps de type IgM seulement, plus courants et naturellement présents dans l’organisme, sont très peu susceptibles de provoquer des réactions graves. On ne sait pas avec certitude si les anticorps IgG à faible titre peuvent provoquer des réactions sévères, car le titrage n’a pas été fait dans tous les cas. La plupart des cas ont été signalés en Asie,17 en particulier à Taïwan et à Hong Kong, et l’anticorps le plus fréquemment identifié était l’anti-GP.Mur. Dans les cas de MHNNF où les IgG avaient été titrés, les titres variaient de 32 à 1 024, et des cas sévères de MHNNF ont été signalés lors de premières grossesses.17 Dans les groupes d’ascendance européenne, GP.Vw est la glycophorine hybride la plus susceptible de stimuler l’anti-Mi(a). Les titres d’IgG déclarés varient de 32 à 128.17La détection des anticorps de glycophorines hybrides (anti-Mi(a), anti-Mur et anti-Vw) pendant la grossesse représente un défi croissant face à la diversification ethnique due à l’immigration mondiale.20, 24 L’anti-Mi(a) a été comparé à l’anti-Kell en termes de fréquence et de niveau de gravité potentielle chez les personnes d’origine asiatique20; cependant, il ne fait pas partie des dépistages systématiques conçus pour les groupes ethniques occidentaux. C'est pourquoi certains fournisseurs préconisent d’inclure dans les épreuves prénatales des cellules porteuses de l’antigène Mi(a), en particulier pour les personnes d’origine asiatique; cependant, à ce jour, les cellules disponibles sur le marché pour le dépistage ne sont généralement pas porteuses de l’antigène Mi(a).20, 24
Les anticorps dirigés contre les antigènes à faible prévalence (y compris les anticorps anti-Mi(a)) doivent être considérés comme une cause possible de MHNNF dans les cas où il existe un ictère néonatal inexpliqué avec un test direct à l’antiglobuline positif et un dépistage des anticorps maternels et/ou néonatals négatif. En particulier s’il y a déjà eu des pertes de grossesse non diagnostiquées (voir figure 3), il est impératif de procéder à un examen supplémentaire des taux d’anticorps de la mère et du père de l’enfant ainsi qu’à un typage antigénique néonatal, généralement par génotypage des antigènes érythrocytaires.20
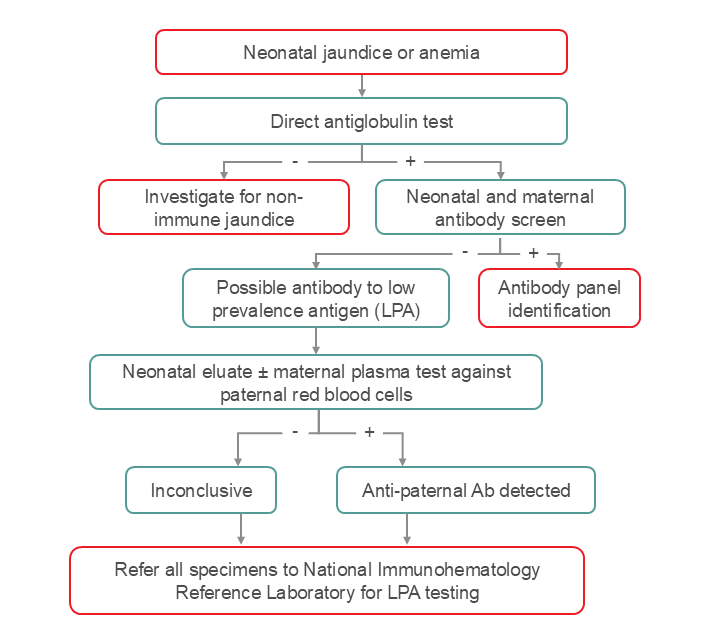
Figure 3. Stratégie de dépistage recommandée pour la recherche d’anticorps dirigés contre un antigène à faible prévalence tel que l’anti-Mi(a). En cas d’ictère néonatal inexpliqué ou d’anémie avec test direct à l’antiglobuline positif, si le dépistage des anticorps est négatif (tant pour la mère que pour le nouveau-né), l’équipe clinique et le personnel de laboratoire doivent envisager la présence d’anticorps dirigés contre un antigène à faible prévalence. Une étape intermédiaire pourrait consister à effectuer une élution des cellules du nouveau-né afin de récupérer les anti-Mi(a) et de comparer le plasma obtenu aux cellules paternelles. Il est également possible d’utiliser le plasma maternel pour le comparer aux cellules paternelles si les anticorps anti-A ou anti-B n’interfèrent pas (c’est-à-dire si les échantillons maternels et paternels sont de groupe identique, ou si les cellules maternelles sont de groupe AB). Le Laboratoire d’immunohématologie national de référence peut réaliser des tests ou fournir des conseils.
Une consultation en médecine materno-fœtale est fortement recommandée si des anticorps dirigés contre les glycophorines hybrides sont connus ou détectés pendant la période prénatale. À l’heure actuelle, il n’existe aucune recommandation sur les titres maternels critiques, mais la revue de Heathcote et coll. semble indiquer que des titres égaux ou supérieurs à 32 augmentent le risque de MHNNF ayant une importance clinique.17
En cas de suspicion d’anti-Mi(a), il convient d’envisager un test génétique des antigènes du groupe sanguin du nourrisson et du père afin d’identifier les glycophorines hybrides, car ceux-ci peuvent avoir une incidence sur les grossesses futures et les besoins transfusionnels.20, 24 Pour les grossesses suivantes ou si le père de l’enfant est porteur de l’antigène, il est recommandé de consulter rapidement un spécialiste en médecine materno-fœtale afin de surveiller l’anémie fœtale et d’envisager un titrage contre les cellules porteuses de l’antigène Mi(a) à intervalles de 2 à 4 semaines.20 Vous pouvez contacter votre service local de médecine transfusionnelle pour organiser le test de dépistage de l’antigène Mi(a) par le Laboratoire d’immunohématologie national de référence.