Author: Sheila O'Brien, PhD
Online publication date: September 2024
Surveillance report 2023
We are pleased to present this annual report describing transmissible blood-borne infection surveillance. High quality and timely surveillance is central to the safety of the blood supply. This includes monitoring of transmissible disease markers that the blood is tested for (including bacteria) and investigation of any reports of possible transfusion transmission, as well as a horizon scan for any new pathogens that may pose a risk.
To request older Surveillance Reports, please contact us through the Feedback form.
Surveillance Report 2023
Executive Summary
The year 2023 marks 25 years since the inception of Canadian Blood Service. This annual report describes surveillance of transmissible blood-borne infections and emerging threats of concern. High quality and timely surveillance are central to the safety of the blood supply. This includes monitoring of transmissible disease markers that the blood is tested for and investigation of any reports of possible transfusion transmission, as well as a horizon scan for any new pathogens that may pose a risk. Non-infectious surveillance of aspects of donor health and safety are also included.
Infectious risk monitoring
The most up-to-date tests for pathogens are used to identify infectious donations and prevent their release for patient use. In 2023, transmissible disease rates per 100,000 allogeneic donations continued to be very low: human immunodeficiency virus (HIV) 0.5, hepatitis C (HCV) 8.8, hepatitis B (HBV) 7.2, human T-cell lymphotropic virus (HTLV) 0.6 and syphilis 10.4. Selective testing of donors at risk of Chagas disease identified 2 positive donations, and there were 10 donations that were positive for West Nile Virus (WNV). Residual risk estimates of a potentially infectious donation from a unit of blood are very low at 1 in 19.7 million donations for HIV, 1 in 41.5 million donations for HCV and 1 in 2.9 million donations for HBV. Lookback and traceback investigations did not identify any transfusion transmitted infections. New to this report are results from HIV, HBV and HCV viral genotyping carried out since 2016. Most HIV positive donations were non-B subtype typically associated with heterosexuals. The most common HBV genotype was D, followed by A, B and C. These genotypes are common in particular parts of the world and likely reflect the diversity of immigrants in Canada. The most common HCV genotypes were 1 and 3. The viral genotypes observed in donors are not dissimilar from the Canadian general population.
Also new to this report is a section on source plasma donations. Source plasma is collected by apheresis to be sent for manufacturing of fractionated plasma products such as intravenous immune gammaglobulins (IVIG) and albumin. In 2023, nearly 100,000 source plasma donations were collected, mostly from repeat donors (95%). Transmissible disease rates per 100,000 source plasma donations were very low: HIV 1.0, hepatitis C (HCV) 4.0, hepatitis B (HBV) 7.0, HTLV 0 and syphilis 7.0. This is comparable to allogeneic donations.
In 2023 pathogen reduction technology was phased in for platelet products all but eliminating the risk of bacterial contamination. By the end of December 2023, in all regions whole blood derived buffy coat platelets were being pathogen reduced and by May 2024 apheresis platelets will also be pathogen reduced. Non-pathogen reduced platelets are distributed on rare occasions, if preferred by clinicians. Bacterial growth was identified in 140 platelet products that had not been pathogen reduced. Of 621 potential peripheral blood stem cell or bone marrow donors tested, none were positive for any infectious marker. Of 283 samples from mothers donating stem cells collected from the umbilical cord and placenta (called “cord blood”) after their babies were born, one was positive for syphilis antibodies.
Since 2014 donors with false reactive/unconfirmed results for HIV, HCV or HBV have been eligible to give a re-entry testing sample after six months, and if all infectious tests are negative, they may re-commence donating. As of Jan. 16, 2023, the donor re-entry program was also extended to donors who had previously tested false reactive/non-confirmed for HTLV or syphilis. As of Dec. 31, 2023, 1,324 donors have been able to donate again and have given over 10,000 donations. Of these, 125 were donors who had previously tested false reactive for HTLV or syphilis who gave 296 donations. The donor re-entry program supports a safe blood system and provides a positive contribution to the active donor base.
Horizon scanning for emerging pathogens monitors potential threats to safety. The risk of a tick-borne disease, babesiosis, continues to be monitored. The parasite (Babesia microti) that causes babesiosis appears to be in the early stages of becoming established in a few places in Canada, especially in Manitoba. Travelers and former residents from malaria risk areas are temporarily deferred for malaria risk. In addition, a three-week deferral for any travel outside Canada, continental USA and Europe reduces risk from short term travel related infections such as Zika virus.
COVID-19
A novel coronavirus (SARS-CoV-2) was identified in Wuhan, Hubei Province, China in November 2019. On March 11, 2020, the World Health Organization declared a COVID-19 pandemic, and on May 5, 2023, declared it over. SARS-CoV-2 is not transmissible by blood. Canadian Blood Services has tested over a million blood samples since May 2020 for SARS-CoV-2 antibodies in collaboration with the COVID-19 Immunity Task Force commissioned by the Canadian Federal Government. Seroprevalence to natural infection increased from less than ~7% in January 2022 to about 83% in December 2023 when the more infectious variant, Omicron and its sub-variants, were dominant. Vaccine related antibodies were found in nearly 100% of donors over 2022 and 2023, consistent with wide scale vaccination programs. Many donors now have antibodies from both vaccination and infection. These results were important for public health officials to plan safety interventions for Canadians.
Sexual behaviour-based screening
On Sept. 11, 2022, the three-month deferral for gay, bisexual, and other men who have sex with men was removed and replaced with deferral based on sexual risk questions that all donors must answer regardless of their gender or sexual orientation. Since implementation, less than 1% of donors were temporarily deferred using these new sexual behaviour-based questions. Four allogeneic and one source plasma donations were HIV positive, with a similar HIV positivity rate as prior to the criteria change. With the stable HIV rates and the low deferral rate, the blood system benefits from the new screening approach.
Donor safety
Adverse reactions to donation are rare. The most common reactions are vasovagal (fainting) and bruising. Vasovagal reactions are more common in first time donors and younger females. Deferrals for low hemoglobin are higher in females than males. The inter-donation interval for whole blood donations in females is longer than for males (84 vs 56 days) to allow more time for females to recoup their body iron levels. In 2023 female donors on their 10th, 20th, or 30th etc., donations were tested for ferritin, a measure of body iron status that can indicate low body iron levels before hemoglobin drops. If ferritin was low (≤ 24 mcg/L), donors were advised not to donate for at least six months and to see their health care practitioner for further testing and advice about iron supplementation. Nearly a quarter (23%) of these female donors had low ferritin in spite of passing their hemoglobin test on donation. Low ferritin was associated with more frequent donation. To ensure ongoing donor iron health, ferritin testing is important for safeguarding donors.
Donor demographics
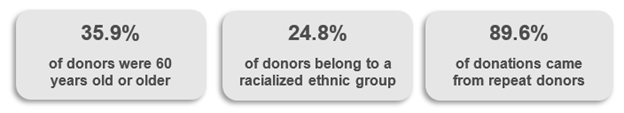
Most donations are from repeat donors. A little more than half of donors are males, over a third (36%) are persons 60 years of age or older. Nearly half (45%) of donations are from Ontario. Based on responses to a voluntary question about race/ethnicity nearly a quarter of donations are from Black, Indigenous and Racialized donors.
Introduction
Safety of the blood supply from pathogens involves a multifaceted approach. Donor education materials on the internet and required reading just before donating explain risk factors for transmissible infections and who should not donate. Before donating blood, everyone must complete a health history questionnaire which includes questions about specific risk factors for transmissible infections. This is followed by an interview with trained staff to decide if the person is eligible to donate blood. All donations are tested for markers of transfusion transmissible agents including HIV, HBV, HCV, HTLV (a rare cause of leukemia) and syphilis. WNV testing is undertaken during the at-risk period of the year (spring, summer, and fall) and in at-risk travelers during the winter. In addition, donors at risk of Chagas disease (which is transmitted by the bite of an insect in Latin America) are tested. Pathogen reduction technology is being phased in for platelet products, but those not pathogen reduced are tested for bacteria. SARS-CoV-2, which causes the respiratory infection, COVID-19, emerged in 2020 but it is not transmitted by blood transfusion. Epidemiologic data on the frequency of SARS-CoV-2 antibodies in the donor population over time provided support for public health decision making during the pandemic.
Surveillance includes monitoring of transmissible infection testing in donors, investigation of possible transfusion transmitted infections in recipients and horizon scanning for new, emerging pathogens. Monitoring the safety of donors is also essential. Although surveillance is conducted in “real time” over each year, final verification steps generally impose a short delay in producing a final report. This report describes Canadian Blood Services’ approach to surveillance of transmissible blood-borne infection, infectious threats, and donor safety, as well as data for the calendar year of 2023.
Blood donor surveillance
The numbers of allogeneic blood donations (whole blood and platelet and transfusible plasma apheresis) from first time and repeat donors are shown in Figure 1. The majority of donations are from repeat donors (89.9%). The decreasing trend in the proportion of donations from first time donors from 11.1% in 2019 to 9.8% in 2020 to 8.3% in 2021 is reversing with 9.5% in 2022 and 10.1% in 2023.
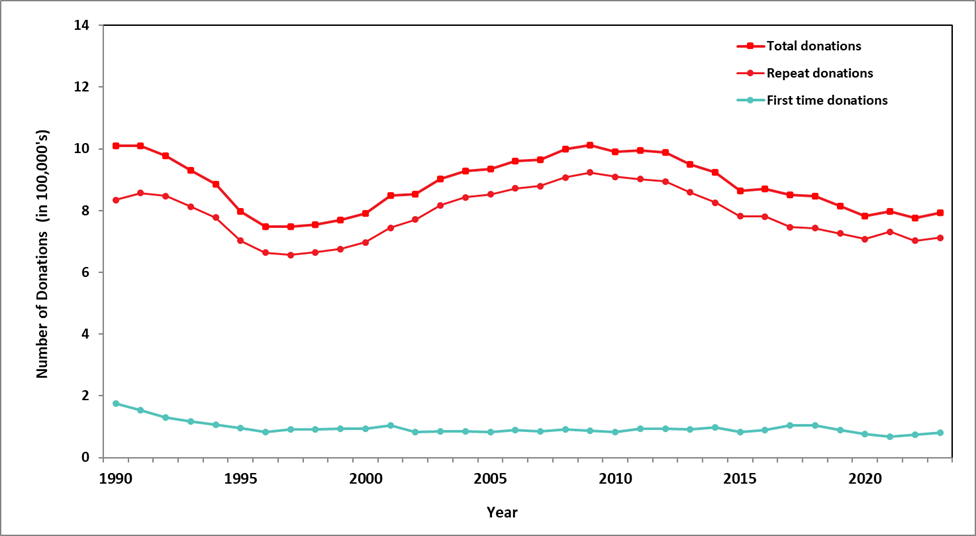
Note: Source plasma donations for preparation of fractionated products are reported separately.
The “classical” pathogens
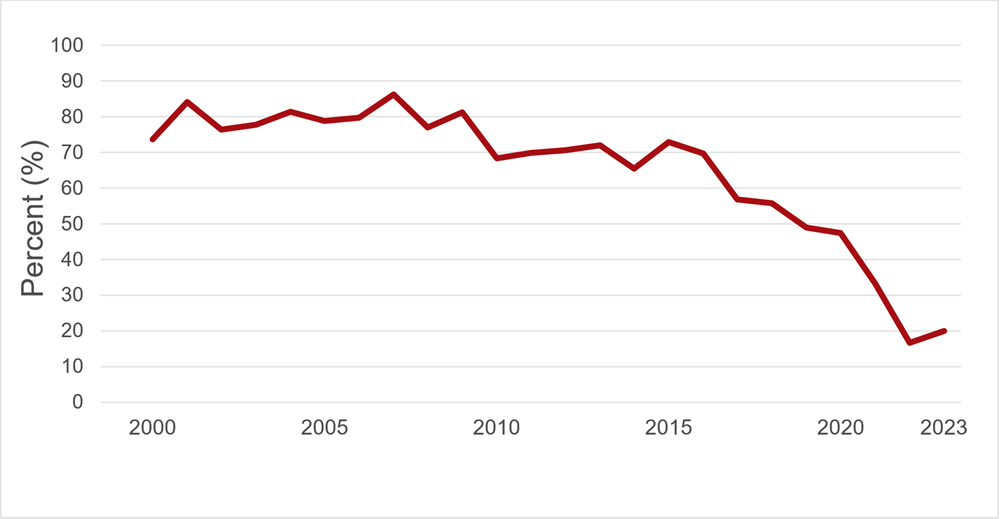
Details of screening tests used, and dates of implementation are shown in Appendix I. In Table 1 the numbers of positive donations and the rates of positive tests per 100,000 donations are shown for 2023 by demographic groups. When a transmissible infection is detected, it is most often in a first time donor as these donors have not been tested previously and may have acquired the infection at any time in their lives. There were four HIV positive allogeneic donations in 2023. In the past five years, the number of HIV positive donations has ranged from zero to four per year. The rate per 100,000 donations has remained stable for HIV and HTLV and the rate for repeat donations is extremely low (see Appendix II). There is a trend towards increased HBV, HCV, and syphilis positive first time donations (for syphilis also in repeat donations). Canada is considered a low prevalence country for HBV and HCV. Both HBV and HCV infections are more common in immigrants from higher prevalence countries where transmission risk from medical procedures and household contacts is higher, as well as sexual contact. Routine HBV vaccination also may not occur in these countries. As Canadian Blood Services works to be inclusive of donors from different ethnic groups and nationalities, a few more HBV and HCV positive donations are not unexpected. Curative treatment for HCV has been available since 2014, and more widely available since 2016 in Canada. As shown in Figure 2 below, the percentage of HCV antibody positive first time donations which are also HCV NAT positive, indicating active infection, has been decreasing. Hence the overall increase in HCV positive first time donations in recent years is likely due to old infections that either resolved spontaneously or were treated. Syphilis (first time and repeat donations) has increased from 4.1 per 100,000 donations in 2019 to 9.8 in 2020, 6.7 in 2021 and 10.7 per 100,000 in 2022 and is holding steady at 10.4 per 100,000 in 2023. Syphilis cases have been increasing in the general population, but rates are not directly comparable because public health cases only include people who had reason to be tested (usually new infections with symptoms) whereas blood donors with syphilis positive test results include both people unaware of their infection and those who may have been infected in the past and cured. It is unlikely that syphilis could be transmitted by transfusion due to modern blood processing methods.
Table 1: Confirmed positive donations and prevalence rates per 100,000 allogeneic donations in 2023.
| Characteristics | Number of Donations |
Percent of Donations |
HIV | HCV | HBV | HTLV | Syphilis | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pos | Rate | Pos | Rate | Pos | Rate | Pos | Rate | Pos | Rate | |||
| Donor Status | ||||||||||||
| First time | 79,996 | 10.09 | 3 | 3.75 | 69 | 86.29 | 57 | 71.28 | 5 | 6.25 | 57 | 71.28 |
| Repeat | 712,294 | 89.91 | 1 | 0.14 | 1 | 0.14 | 0 | - | 0 | - | 25 | 3.51 |
| Gender | ||||||||||||
| Female | 329,296 | 41.56 | 0 | - | 19 | 5.77 | 13 | 3.95 | 3 | 0.91 | 17 | 5.16 |
| Male | 462,964 | 58.44 | 4 | 0.86 | 51 | 11.02 | 44 | 9.50 | 2 | 0.43 | 65 | 14.04 |
| Age | ||||||||||||
| 17-29 | 116,847 | 14.75 | 1 | 0.86 | 0 | - | 8 | 6.85 | 0 | - | 18 | 15.40 |
| 30-39 | 144,524 | 18.24 | 2 | 1.38 | 18 | 12.45 | 8 | 5.54 | 3 | 2.08 | 24 | 16.61 |
| 40-49 | 142,660 | 18.01 | 1 | 0.70 | 12 | 8.41 | 13 | 9.11 | 1 | 0.70 | 17 | 11.92 |
| 50+ | 388,229 | 49.00 | 0 | - | 40 | 10.30 | 28 | 7.21 | 1 | 0.26 | 23 | 5.92 |
| Total | 792,260 | 100 | 4 | 0.5 | 70 | 8.84 | 57 | 7.19 | 5 | 0.63 | 82 | 10.35 |
Note: 7 anti-HCV of the 70 positive donations had confirmatory testing just above the lower cut-off suggestive of infections resolved in the distant past or false positive tests
All transmissible infection positive donations are destroyed. The main source of risk is when a blood donor acquired the infection too recently to be detected by testing. This is called the “window period” of infection. With current testing the window period is very short. For HIV and HCV, an infection would be detected within one to two weeks of a donor being infected by nucleic acid testing (NAT) and for HBV within one month. The residual risk of infection is the estimated risk of a potentially infectious donation being given during the “window period”. These estimates, shown in Table 2, are based on new infections (positive donations with a prior donation which tested negative within the last three years) and include 2019 -2022 data. The risk is currently extremely low, but of course it can never be zero.
Table 2: Estimated residual risk of HIV, HCV, and HBV.
| HIV | HCV | HBV |
|---|---|---|
| 1 in 19.7 million donations | 1 in 41.5 million donations | 1 in 2.9 million donations |
Risk factors
Risk factor interviews are carried out with donors who test positive for transmissible infections. The main risk factors are shown in Table 3. HIV infections are very rare in donors; therefore, it is difficult to generalize the risk factors. It should be noted that participation is voluntary and therefore there is only data for some donors, and that for many donors no risk factors were identified.
Table 3: Risk factors for infectious disease in blood donors.
| Infection | Risk Factor |
|---|---|
| HIV | High risk heterosexual partners Male to male sex |
| HCV | History of intravenous drug use History of blood transfusion (prior to testing) Been in prison Born in a higher prevalence country |
| HBV | History of living with someone who has hepatitis Ethnic origin from a higher prevalence country Born in a higher prevalence country |
| HTLV | History of other sexually transmitted disease History of blood transfusion Born in a higher prevalence country |
| Syphilis | Previous history of syphilis Male to male sex Sex with an intravenous drug user Born in a higher prevalence country |
Note: Not all donors are available/willing to be interviewed.
Viral genotypes
Since February of 2016 all donations positive for HIV, HBV, or HCV for which there was sufficient sample were genotyped by the Public Health Agency of Canada (PHAC) National Microbiology Laboratory in Winnipeg. Genotyping is important to understand when new or rare genotypes appear in the donors which may suggest a shift in risk factors. The genotypes in all tested samples are shown in Table 4. In Canada most HIV-1 infections were subtype B historically, but this has been changing over the last 10 to 15 years. Note that national genotyping reports by PHAC can lag by at least five years. In heterosexuals the non-B subtypes are more common, hence the high proportion of non-B subtypes (60%) in donors may reflect the former deferral of gay and bisexual men who have sex with men over the period. Different HBV genotypes are more common in different parts of the world. For example, HBV genotype A is more common in Africa and northern Europe, B and C are more common in Asia, and D in Africa, Europe, the Mediterranean and India. Hence the diversity of HBV genotypes in donors may reflect the diversity of immigrants in Canada. HCV genotype 1 (sub-types 1a and 1b) is the most common in Canada as is also seen in the donors. HCV sub-genotype 3a is the next most common in Canada, also seen in the donors.
Table 4: Genotypes of HIV, HBV, and HCV positive donations 2016-2023
| Genotype | Number of samples | Percentage |
|---|---|---|
| HIV | ||
| 01-AE | 1 | 10% |
| A1 | 1 | 10% |
| B | 4 | 40% |
| C | 3 | 30% |
| F1 | 1 | 10% |
| Total | 10 | |
| HBV | ||
| A | 89 | 26.6% |
| B | 75 | 22.4% |
| C | 38 | 11.3% |
| D | 123 | 36.8% |
| E | 8 | 2.4% |
| G | 1 | 0.3% |
| Total | 334 | |
| HCV | ||
| 1 | 1 | 0.7% |
| 6 | 1 | 0.7% |
| 1a | 57 | 43.5% |
| 1b | 33 | 25.2% |
| 2a | 5 | 3.8% |
| 2b | 8 | 6.1% |
| 3a | 20 | 15.3% |
| 3g | 1 | 0.7% |
| 3i | 1 | 0.7% |
| 4a | 3 | 2.3% |
| 4n | 1 | 0.7% |
| Total | 131 |
Chagas disease (Trypanosoma cruzi)
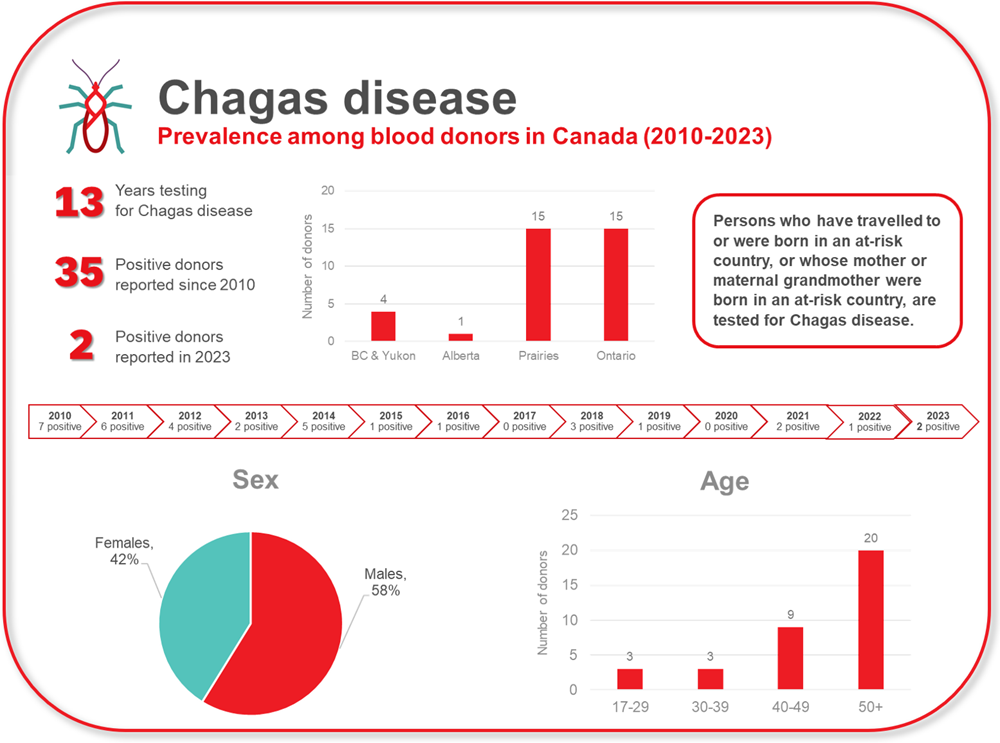
Chagas disease is caused by infection with a parasite called Trypanosoma cruzi (T. cruzi). The reduviid insect that carries the parasite is found in parts of Mexico, Central and South America. Infection can occur following a bite by the insect, be passed on from mother to child during pregnancy, or passed on by blood transfusion. Donors are deferred if they have had Chagas disease. In addition, potential donors are asked whether they, or their mother or maternal grandmother, were born in Mexico, Central America, or South America, and if they have spent a total of six consecutive months or more in Mexico, Central America, or South America. Since testing of at risk donors was implemented in 2010, 35 T. cruzi positive donations have been identified (See Figure 3). In 2023, there were two positive donations of 7,863 tested. Notably, no donor with only travel risk (not born in a risk area or mother/grandmother born in a risk area) has ever tested postive.
West Nile Virus
West Nile Virus (WNV) is a mosquito-borne virus that has been present in North America since 1999 (in Canada since 2002). Although symptoms can be severe, they are usually mild, and most people are not aware of their infection. During spring, summer, and fall, donations are routinely tested in mini pools of six donations. However, to further reduce the risk, an algorithm is applied to identify all donations from areas where West Nile Virus is active, and these are tested as single donations. In 2023, 407,624 donations were tested over the spring, summer, and fall when all donations were tested, and 10 donations were positive, identified from Aug. 8 to Sept. 19. Six positive donations were from Ontario, three from Alberta, and one from the Prairies region. The geography of general population cases was similar. With only travelers tested over the winter, 61,658 donations (from Jan. 1 to May 28, 2023, and Nov. 26 to Dec. 31, 2023) were tested and none found to be positive. Note: follow up testing and interviewing of WNV-positive donors by local public health may classify some of these donors into categories other than asymptomatic. The low infection rate in 2023 WNV is similar to previous years.
Source plasma surveillance
Source plasma is plasma collected by apheresis to be sent for manufacturing of fractionated plasma products such as immunoglobulin and albumin. Because the demand for fractionated products, especially immunoglobulin, is increasing, Canadian Blood Services is collecting more source plasma. In 2023, there were eight plasma donor centres (PDCs) operating across Canada, of which three were opened during 2023. There were also seven whole blood focused centres that collect source plasma. Roughly two thirds of source plasma donations were collected at PDCs (operating in British Columbia, Alberta, and Ontario), with the remainder at centres that collect both whole blood and plasma. By region, the distribution of source plasma donations ranged from 8.4% in the Atlantic provinces to over 41% in Ontario. The majority of these were from repeat donors (95%) and about two thirds from males. Figure 4 shows the locations of PDCs and Figure 5 shows the number of donations (includes both PDC and other site source plasma) by donation status. Source plasma may currently be donated once a week.
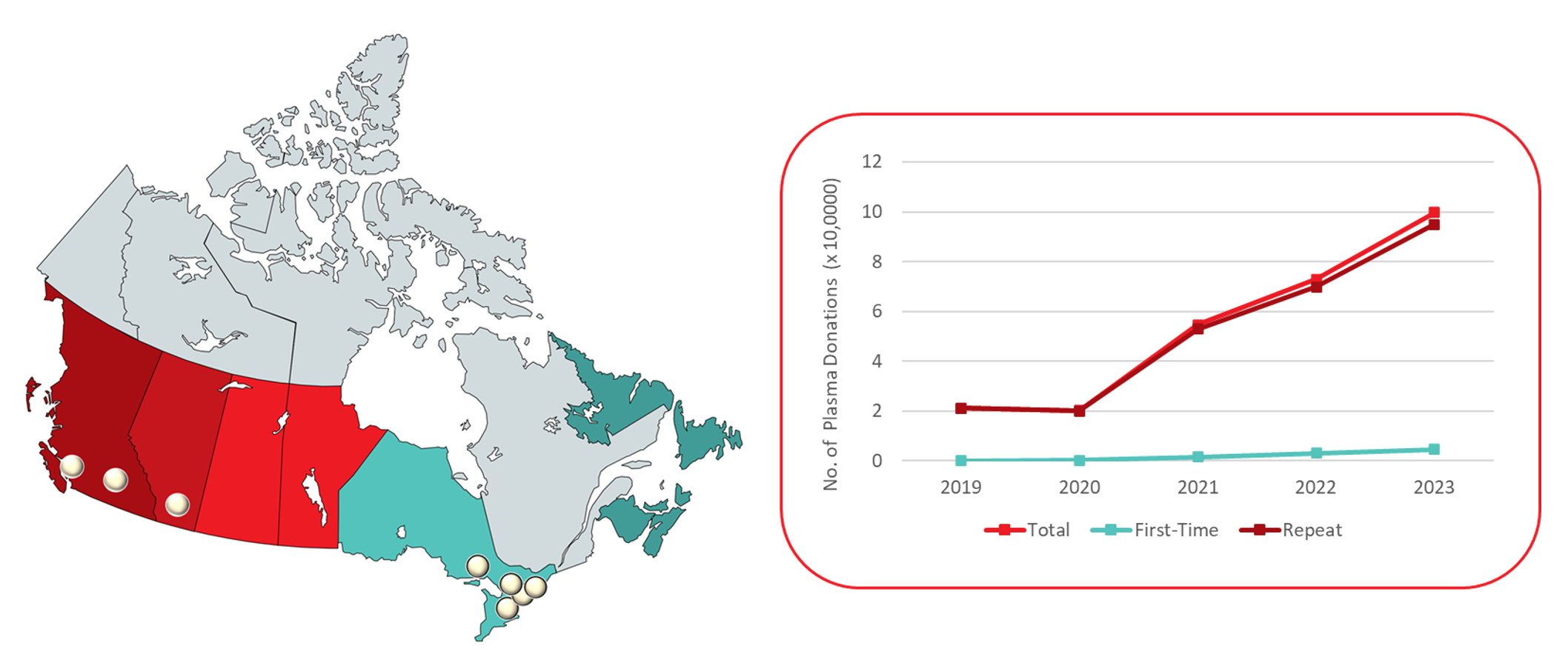
Source plasma donations increased almost fourfold between 2019-2023, to almost 100,000 donations in 2023. Additionally, although first time donations constituted a small proportion of the total (5%), the number of first time source plasma donors per year increased from 81 to over 4,500 over this period. This reflects increasing recruitment of new donors for plasma donation rather than recruiting from those already donating whole blood. In this report, donors were classified as “first time” based on the first time they ever donated any type of donation.
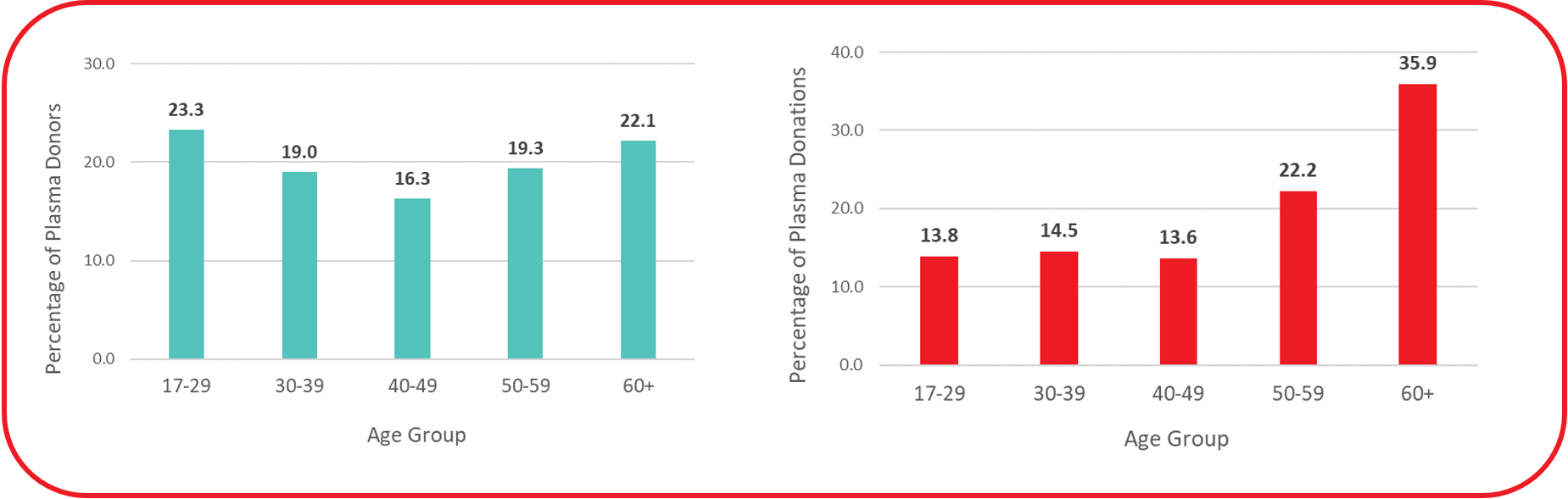
Figure 6: Proportion of plasma donors and donations by age group.
While almost a quarter of source plasma donors in 2023 were aged 17-29 years old, they gave only about 14% of donations (see Figure 6). Conversely, about 22% of donors were aged 60 years or older but accounted for over 35% of all donations. Caucasian donors made up 73.7% of source plasma donors. Based on voluntary self-reporting of race/ethnicity, donors identifying as belonging to a Racialized group ranged from 12.3% in the Atlantic provinces to 33.4% in Ontario.
Table 5 shows the numbers of positive donations and the rates of donations testing positive per 100,000 plasma donations for 2023. There was one HIV positive donation in 2023, four HCV positive donations, seven HBV positive donations, and seven syphilis positive donations. When a transmissible infection is detected, it is most often in a first time donor as these donors have not been tested previously. These findings are comparable to whole blood donations.
All donations that tested positive for any transmissible disease marker were destroyed. Because the manufacturing process of source plasma includes several pathogen reduction steps, the risk of transmission of any of these infections is close to zero.
Table 5: Confirmed positive donations and prevalence rates per 100,000 source plasma donations in 2023
| Characteristics | Number of donations |
Percent of donations |
HIV | HCV | HBV | HTLV** | Syphillis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pos | Rate | Pos | Rate | Pos | Rate | Pos | Rate | Pos | Rate | ||||||
| Donor status | |||||||||||||||
| First time* | 4,595 | 4.61 | 0 | - | 3 | 65.29 | 6 | 130.58 | 0 | - | 2 | 43.53 | |||
| Repeat | 95,052 | 95.39 | 1 | 1.05 | 1 | 1.05 | 1 | 1.05 | 0 | - | 5 | 5.26 | |||
| Gender | |||||||||||||||
| Female | 37,189 | 37.32 | 0 | - | 1 | 2.69 | 1 | 2.69 | 0 | - | 2 | 5.38 | |||
| Male | 62,458 | 62.68 | 1 | 1.60 | 3 | 4.80 | 6 | 9.61 | 0 | - | 5 | 8.01 | |||
| Age | |||||||||||||||
| 17-29 | 13,787 | 13.84 | 1 | 7.25 | 0 | - | 2 | 14.51 | 0 | - | 2 | 14.51 | |||
| 30-39 | 14,445 | 14.51 | 0 | - | 2 | 13.84 | 1 | 6.92 | 0 | - | 3 | 20.75 | |||
| 40-49 | 13,577 | 13.63 | 0 | - | 1 | 7.37 | 1 | 7.73 | 0 | - | 0 | 0.00 | |||
| 50+ | 57,828 | 58.03 | 0 | - | 1 | 1.73 | 3 | 5.19 | 0 | - | 2 | 3.46 | |||
| Total | 99,647 | 100 | 1 | 1.00 | 4 | 4.01 | 7 | 7.02 | 0 | - | 7 | 7.02 | |||
* Donors were classified as "first time" based on the first time they ever donated any type of donation.
** PDC sites do not test for HTLV
Donor re-entry program
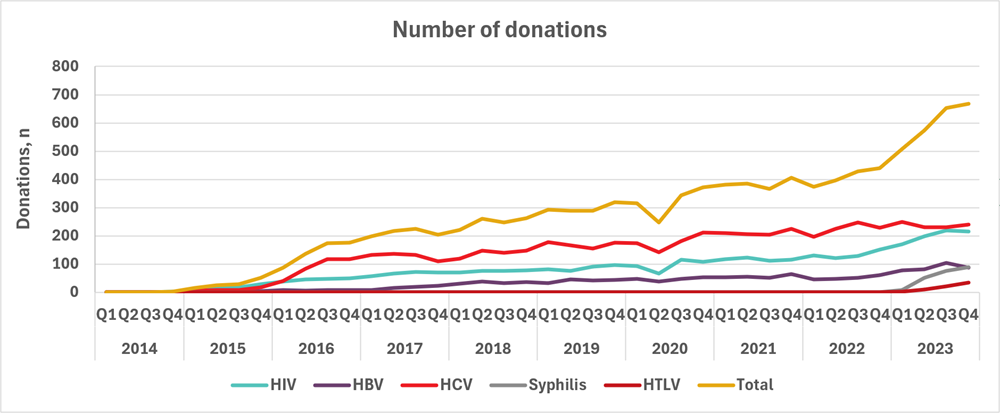
In 2014, Canadian Blood Services established a donor re-entry program for donors who had been indefinitely deferred for HIV, HBV, HCV serological markers that were false reactive/non-confirmed and false reactive nucleic acid tests for HIV, HCV, and HBV. As of Jan. 16, 2023, the donor re-entry program also included syphilis and HTLV. These false reactive/non-confirmed results occur because very sensitive tests are used for blood screening and sometime react to non-infection related components in the blood. Donors with false reactive/non-confirmed results are now coded as eligible for donor re-entry and are deferred for six months. They are eligible to return any time after six months since their last donation to provide an initial specimen-only sample to be re-tested. Donors who are negative for all routinely screened infectious disease markers are then eligible to return to donate blood products. Donors are informed of the donor re-entry program in the donor notification letter that is sent to them following their reactive/non-confirmed test result. Figure 7 shows the number of donors who were eligible, tested, and donated from Feb. 3, 2014, to Dec. 31, 2023, for HIV, HBV, and HCV and from Jan. 16, 2023, to Dec. 31, 2023, for syphilis and HTLV false reactive/non-confirmed tests and the total. Just over 1,300 donors have been able to donate again and have given over 10,000 donations.
However less than 25% of donors eligible for re-entry return to provide an initial specimen-only sample. Between half and three quarters of donors are eligible to donate after their specimen is tested depending on the marker and most return to donate (82% overall). Figure 8 shows the increasing trend in the number of donations per quarter from 2014 to 2023 from donors who re-entered the donor pool.
Surveillance for emerging pathogens
A horizon scan of potential blood borne infections (e.g., Mpox (monkeypox), tick-borne pathogens) and agents that can negatively impact donor health or the ability to collect blood products (e.g., avian influenza) in the general community ensures rapid revision of donor policies to maintain safety. Even before a new infectious disease is reported in Canada, we are aware of emerging infectious agents by monitoring outbreaks in other parts of the world. As international travel becomes more frequent post-COVID-19 pandemic, it is important to be vigilant as infections can rapidly enter from other countries. To ensure that potential risks are identified, Canadian Blood Services needs to be connected with the latest infectious disease, public health and microbiology information at all times. Canadian Blood Services’ medical and scientific staff participate in public health and infectious disease professional organizations and monitor web sites and journals where new information is posted. When appropriate, the Alliance of Blood Operators (ABO) Risk Based Decision Making Framework can be used to facilitate policy decision making. This ensures that relevant assessments including infectious risk to recipients, operational impact of strategies, stakeholder input and health economics are considered. A range of infectious agents that could emerge as a threat within Canada are being monitored.
Additionally, current testing and donor deferral policies are re-evaluated to see if they remain appropriate or are resulting in unnecessary donor deferrals without enhancing safety.
Coronavirus disease 2019 (COVID-19)
SARS-CoV-2 is a novel coronavirus first identified in Wuhan, Hubei province of China in late 2019. It is responsible for a severe respiratory illness known as the 2019 coronavirus disease (COVID-19). Some people become extremely ill and can die from complications, while others experience mild symptoms and may not be aware of their infection. On March 11, 2020, the World Health Organization declared COVID-19 to be a pandemic. Since then, nearly five million Canadians have received a COVID-19 diagnosis and nearly 60,000 people have died. The pandemic was declared over on May 5, 2023.
SARS-CoV-2 (COVID-19) seroprevalence
Early in the pandemic with less than 500 confirmed cases across Canada (March 23, 2020), strict physical distancing measures were implemented in most provinces. As a result, the first wave of the epidemic peaked by the end of April and plateaued in July and August 2020. Multiple waves fueled by new variants followed. In December of 2021 cases of the Omicron variant appeared. Vaccination of Canadians began in December 2020 and was rolled out over 2021 (2 shots). Starting in November 2021, some Canadians became eligible for a third dose, and in 2022 some people could also have a fourth shot. About 89% of adults have received at least the primary series (2 shots). SARS-CoV-2 vaccination is now seasonal, but as of December 2023 less than 17% of Canadians had received a fall booster shot.
Public health reported cases do not show the true infection rate because some infections will not cause illness, others may not be severe enough for people to seek testing, and testing may be disproportionately directed to outbreaks. Importantly, when the Omicron variant arrived in late 2022, public health testing was overwhelmed by large numbers of cases and limited testing was only available to certain groups such as high-risk individuals. Over 2022, spikes in infection activity were identified with wastewater surveillance, but only seroprevalence could quantify the proportion of people infected. Testing for SARS-CoV-2 antibodies was important to understand what proportion of the population had already been infected (the seroprevalence), what proportion have vaccine related antibodies and to monitor infection over the course of the pandemic. These data were used to improve mathematical models to predict the course of infection, understand population immunity, and inform public health policy. In partnership with the COVID-19 Immunity Task Force, Canadian Blood Services tested residual blood for SARS-CoV-2 antibodies from April 2020 to December 2023. As of Dec. 31, 2023, over 1,000,000 samples from across Canada had been tested, of which about 380,000 were in 2023. Two types of antibodies were tested for: antibodies to nucleocapsid due to infection, and antibodies to spike protein due to vaccination or infection. With the roll out of the primary vaccine series in early 2021, antibodies to the spike protein were mainly caused by vaccination and increased to 90% in June 2021. Seroprevalence due to natural infections (nucleocapsid antibodies) remained low up to December of 2021 at about 7% but with the advance of Omicron and its subvariants seroprevalence increased to about 83% by December 2023. The continued near 100% of donors with spike protein antibodies reflected both infection and vaccination antibodies. Figure 9 shows the changes from April 2020 to December 2023.
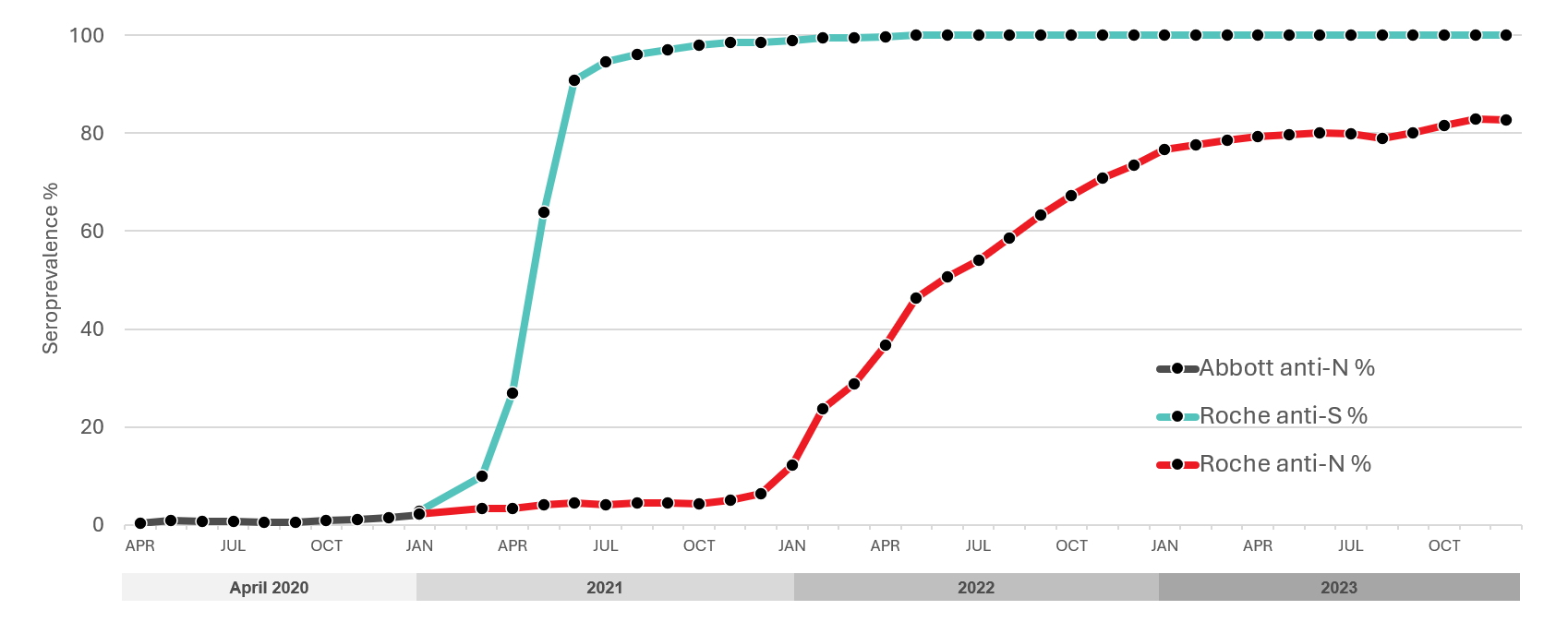
Worldwide, blood services have leveraged their operational capacity to inform public health. In Canada there were significant variations in SARS-CoV-2 seroprevalence by regions, Racialized individuals, and socioeconomic factors. Canadian Blood Services is committed to continuing to play a pivotal role in helping authorities to inform public health policies in the post-COVID-19 era.
Babesiosis
Babesiosis comes from the bite of the black-legged tick (Ixodes scapularis) which can transmit the parasite B. microti. Usually it causes mild flu-like symptoms, and many people are not even aware that they have had it. However, it can also be transmitted by blood transfusion, and infection in blood recipients can result in severe illness or death. To date babesiosis cases in the general population have been reported mainly in the Northeastern and Upper Midwest parts of the United States where more than 1,500 cases per year are reported. Cumulatively more than 200 infections in the United States are believed to have been acquired from a transfusion. In Canada there has only ever been one case of transfusion transmitted babesiosis in 1998 from a donor who had travelled to the Northeast US. In Canada the parasite is found in small numbers of ticks. A 2013 study at Canadian Blood Services and Héma-Québec tested 13,993 blood donations and none were positive. In 2013 one non-donor infected from a tick bite in Canada was reported. Ongoing public health surveillance of ticks suggests no increase in risk, but a donor study was carried out in 2018 involving more donors. One of 50,752 samples tested from southerly areas across Canada was positive by B. microti nucleic acid testing (NAT, donated in Manitoba and indicating an active infection), and of 14,758 samples tested for antibody to B. microti, four in Southwestern Ontario were positive (but negative by NAT, indicating likely resolved infection at some time in the past). The 2018 rates of babesia in blood donors is several logs lower than the regions in the US which are mandated by the US FDA to test blood donations for babesia NAT. The estimated risk of a clinically relevant infection being transmitted from a blood transfusion in Canada is very low at 0.08 per year (0 – 0.38) per year, or about 1 in 12.5 years. The ABO Risk Based Decision Making Framework was used to evaluate possible risk mitigation strategies. Further surveillance studies are planned to follow babesia and other tick-borne species in Canada.
Travel related infections
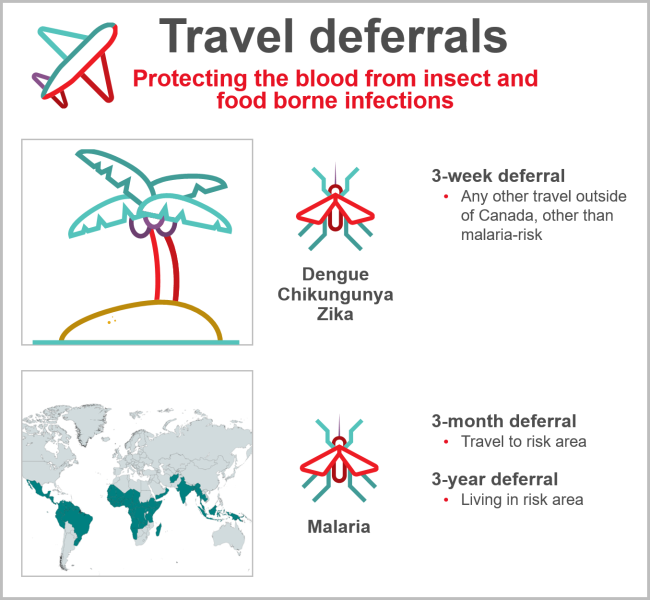
Donors who travel may return with infections that could be transmitted by blood (see Figure 10). Most are only at risk for a period of time after returning until the infection is eliminated from the donor’s bloodstream. Malaria risk is present in parts of the Caribbean, Mexico, Central and South America, Asia, and Africa. Donors are deferred after travel to risk areas for three months, enough time to develop symptoms and self-defer from donation. Former residents of endemic areas are deferred for three years because there is a chance they may be infected for a longer time period without symptoms. Other tropical mosquito-borne infections such as dengue virus have long been present in sunny destinations frequented by Canadians, but in recent years there have been outbreaks of others such as Chikungunya virus and Zika virus not previously seen in the Caribbean, Mexico, Central and South America. Risk to the blood supply was determined to be very low based on quantitative risk assessment. However, to address future travel risks, since 2016 Canadian Blood Services defers all donors who have travelled anywhere outside of Canada, continental US, or Europe for three weeks after travel.
Variant Creutzfeldt-Jakob Disease (vCJD)
vCJD is acquired from eating infected meat (Bovine Spongiform Encephalitis, or BSE, known as “Mad Cow Disease”). Residency or cumulative travel to locations considered a risk for variant Creutzfeldt-Jakob Disease (vCJD) used to result in permanent deferral. These deferrals were first put in place in 1999 and modified over time. Internationally there was a peak of 30 annual cases in 2000, with a steady decline since then, and no community acquired cases since 2016. vCJD travel-related deferrals to all countries were lifted on Dec. 4, 2023. Canadian risk modeling, performed by Canadian Blood Services and Héma-Québec, showed a risk of disease transmission of less than 1 in 16 million. The extremely low risk is due to both the sharp decline in vCJD cases and changes in blood processing methods (removal of white blood cells, known as leukoreduction) which reduces possible transfusion transmission.
Bacteria
Bacteria in blood products usually come from the skin of donors during their blood donation, although occasionally they may come from the donor’s bloodstream. The number of bacteria is usually very low, but because platelet products are stored at room temperature, bacteria can multiply to reach high concentrations and then pose a serious risk to the recipient. Until December 2021, Canadian Blood Services tested 100% of apheresis and pooled platelet products for bacteria using the automated BACT/ALERT culture system in which a sample from the product is inoculated into an aerobic (presence of oxygen) culture bottle and an anaerobic (absence of oxygen) culture bottle and monitored for growth for seven days. If any bacterial growth is detected in the culture bottles, the product is not issued if it is still in inventory at Canadian Blood Services. If it has been sent to a hospital, it is recalled and returned to Canadian Blood Services from the hospital blood bank (i.e., has not been transfused or discarded). In January 2022, Canadian Blood Services started a phase-in implementation of pathogen reduction of platelet products using the INTERCEPT technology, which inactivates bacteria that may be present. This has reduced the number of platelet units tested for bacterial contamination with the BACT/ALERT system. As of December 2023, nationally whole blood derived buffy coat pooled platelets were treated with INTERCEPT, and in May 2024 national pathogen reduction of apheresis platelets was completed. Non-pathogen reduced platelets are distributed on rare occasions, if preferred by clinicians. In 2023, 83,654 platelet products (16,023 apheresis and 67,631 pooled products) were tested, of which 97 apheresis and 325 pooled products had initial positive results for bacterial growth in the culture bottles. From these, seven and 80 cultures were confirmed as true bacterial contaminations, for apheresis and pooled products, respectively. In addition, 10 apheresis and 43 pooled products with initial positive results were not confirmed as they were issued and/or transfused. This represents 140 products in total (16.7 per 10,000) with a chance of bacterial contamination with current testing, including both true positives and suspected positives. The rate is similar compared to previous reporting periods, although the number of products with potential bacterial contamination is less as fewer needed to be tested.
Lookback/Traceback
All cases of potential transfusion transmission of infections are investigated. The Lookback/Traceback Program is notified when a donor tests positive for a transmissible infection, or if the donor reports a transfusion transmissible infection after donating (even if it is not one that would normally be tested for). A Lookback investigation is initiated when previous or historical donations are identified as positive for an infection and hospitals are asked to contact the recipients of these donations to arrange testing. A Traceback is initiated when a recipient is found to have a transmissible infection and transfusion was confirmed and it is queried as to whether their infection could have been from their blood transfusion. Hospitals provide a list of all blood products that the recipient received, and Canadian Blood Services attempts to contact the donors of these products to arrange testing unless current test results are available.
In 2023, there were 15 Lookback cases that required investigation: 11 HCV, 2 HIV, 2 HBV. Ten of these were from donors who had a positive transmissible disease marker and five were from external testing or public health notification. Of these, 12 cases were closed (all recipients that could be contacted were tested). The remaining three cases were still open at the end of 2023. There were four cases from the previous year closed in 2023. No closed cases were associated with transfusion transmission since all recipients had negative test results.
There were 10 Traceback cases that required investigation received from external sources in 2023 (8 HCV, 2 HBV). Of these, nine cases were closed (all donors that could be contacted were tested), and one remains open. There were four cases from previous years closed in 2023. No closed cases were associated with transfusion transmission, since tested donors had negative test results.
Blood stem cells
Blood stem cells can multiply to renew themselves; the new cells develop into blood cells such as red cells, white cells, and platelets. In adults, they are found mainly in the marrow of large bones, with a few cells in the bloodstream. The cord blood of newborn babies, taken from the umbilical cord and placenta after the delivery of a healthy baby, is also very rich in stem cells. Blood stem cells can therefore be obtained from the bone marrow, from circulating blood (called peripheral blood stem cells) or from the umbilical cord (cord blood) after a baby is born. Blood stem cells are very important in treating various diseases such as leukemia, lymphoma, and multiple myeloma. Canadian Blood Services has a coordinated national stem cell program which includes adult registrants and banked cord blood units. Infectious disease testing for all stem cell products includes the same markers tested for whole blood donations.
Canadian Blood Services Stem Cell Registry
Canadian Blood Services Stem Cell Registry is a registry of Canadians who have volunteered to donate either bone marrow or peripheral blood stem cells should a recipient need it at some time in the future. Potential registrants complete a questionnaire which includes risk factors for transmissible infections and are tested for their Human Leukocyte Antigen (HLA) profile. In 2023, there were about 444,600 registrants in the registry. In total, 621 registrants were identified as potential matches for recipients and had additional testing. There were no infectious disease positive results.
Canadian Blood Services Cord Blood Bank
Canadian Blood Services Cord Blood Bank collected cord blood at four sites in Canada in 2023 (Ottawa, Brampton, Edmonton, and Vancouver). Participating mothers at these hospitals who volunteer to donate their baby’s cord blood complete a questionnaire about medical conditions that could be passed on to a recipient, as well as risk factors for transmissible infections. If the donation is suitable for transplantation (i.e., has enough stem cells) with negative results for all infections, the cells are frozen and stored until a recipient needs them. In 2023, there were 283 blood samples from mothers tested and one had a positive test result for syphilis.
Transitioning from male-to-male sex deferral to sexual behaviour-based screening
In the 1980’s men who had sex with another man, even once since 1977, were not eligible to donate blood to reduce the risk of HIV transmission. With much improved donor testing and surveillance for emerging pathogens, the deferral period has been gradually reduced, moving to five years in 2013, to one year in 2016, and to three months in 2019. HIV rates did not increase following any of these changes. Anonymous donor compliance surveys showed that shortening the deferral period allowed more gay, bisexual, and other men who have sex with men to donate blood and donor compliance was not adversely affected. With the three month deferral in place, the risk of releasing an HIV infectious unit for transfusion was very low at 1 in 19.9 million donations (95% CI 1 in 2.7 million – 1 in 1,668 million).
Many countries have switched from lifetime to shorter time deferrals, and recently a number of countries have removed their time-based deferrals in favour of individual behaviour-based screening. The UK removed their time-based deferral in 2021, France removed their time-based deferral in 2022, the Netherlands in 2023, all with different risk reducing donor eligibility criteria. The US also removed their time-based deferral in 2023 and implemented behaviour-based screening.
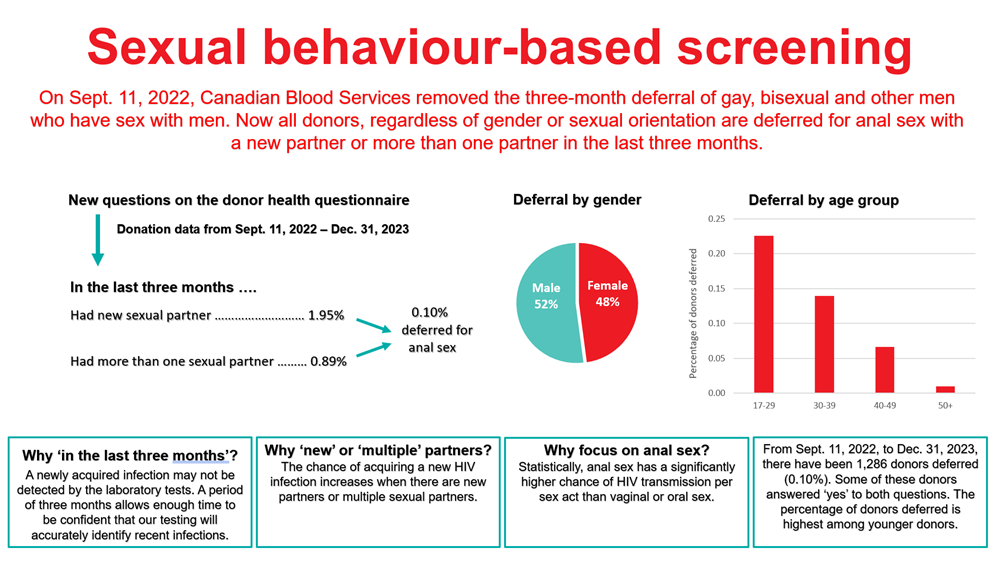
On Sept. 11, 2022, Canadian Blood Services removed the three month deferral for men having had sex with another man and implemented two sexual behaviour-based screening questions that all donors must answer irrespective of sexual orientation or gender (see Figure 11). Additionally, since all donors are asked the same sexual risk questions, trans donors, whose gender identity is different than their sex determined at birth, can register and complete the questionnaire in their affirmed gender. As of Dec. 31 2023, 0.10% of donors presenting to donate were temporarily deferred based on these sexual risks (see Figure 11). Deferred donors tended to be younger and were slightly more likely to be males than females. There were four allogeneic donations that tested positive for HIV during this period, and one source plasma donation. A longer observation period is necessary to see if there has been any change in HIV rates.
Donor safety
Donor reactions
Canadian Blood Services takes many precautions to make sure that giving blood is safe for donors. These include a health screening questionnaire and a hemoglobin fingerstick screen, as well as providing water and a salty snack pre-donation, refreshments post-donation, and monitoring the donor for adverse reactions during and after donation. Most donors do not have any problems during or after their donation, but it is important to keep track of any incidents that happen so that donor care can be improved. Definitions of reactions are shown in Table 6.
Table 6: Reaction and definitions
| Reaction | Definition |
|---|---|
| Vasovagal Moderate Severe |
Donor loses consciousness (faint reactions) Unconscious less than 60 seconds and no complications Unconscious more than 60 seconds or complications |
| Major cardiovascular event | Chest pain or heart attack within 24 hours of blood donation, may or may not be related to donation. |
| Re-bleed | The phlebotomy site starts to bleed after donation. |
| Nerve irritation | Needle irritation or injury of a nerve during phlebotomy. Usually described as sharpshooting pain, arm tingling or numbness. |
| Inflammation/infection | Redness or infection at the needle site, usually seen several days after donating. |
| Local allergic reaction | Rash from skin cleaning solution or dressing, with raised vesicles on the skin. |
| Arm pain | Usually due to blood pressure cuff, tourniquet, or arm position. |
| bruise/hematoma | Temporary dark colour of the skin due to blood leakage from blood vessel at time of phlebotomy. |
| Arterial puncture | Needles inserted in an artery instead of a vein. |
Reaction rates per 10,000 whole blood donations in 2023 are shown in Figure 12 with those in 2022 for comparison. Moderate and severe vasovagal reactions were not significantly different (p>0.05). People more likely to experience a reaction are first time donors, young donors (17-25 years old), and female donors. The reaction reporting system is oriented towards capturing moderate and severe reactions. Most reactions are mild, such as feeling faint or bruising at the needle site, but these are only recorded if mentioned by the donor at some point after donation. A further breakdown of fainting reactions (both moderate and severe) in whole blood donors, by sex and donation history, is provided in Table 7.
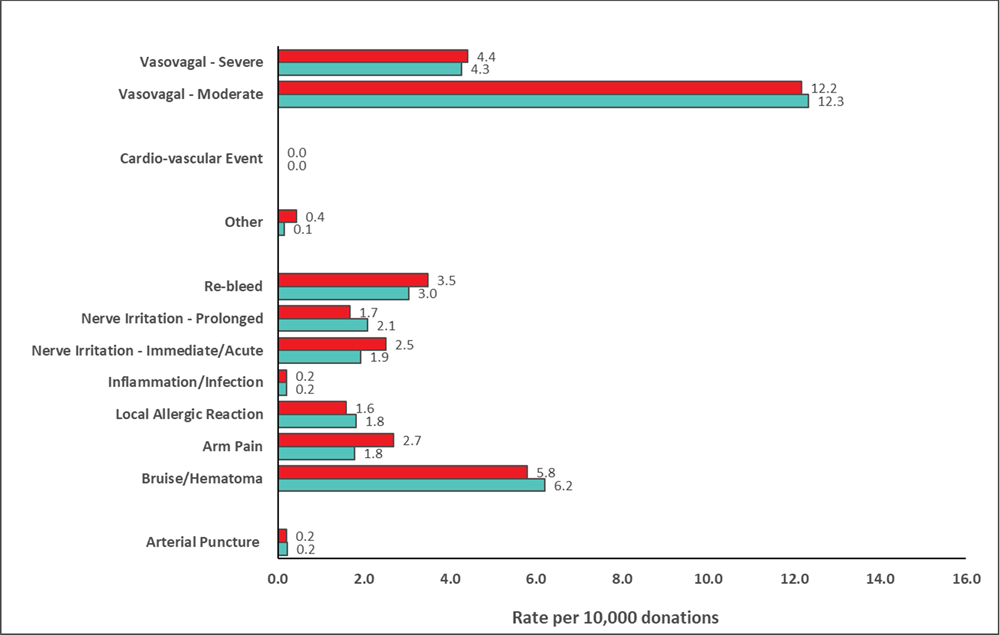
Table 7: Fainting (vasovagal) reactions (per 10,000 collections) in 2023
| Moderate & Severe (all)* | Associated with injury* | |||
|---|---|---|---|---|
| Donation status | Male | Female | Male | Female |
| First Time | 45.8 | 85.4 | 2.1 | 35 |
| Repeat | 6.1 | 20.1 | 0.2 | 0.6 |
Injuries were generally not severe such as bruises and cuts from falling.
*All comparisons (male vs female, first time vs repeat) are statistically significant among moderate & severe reactions combined (p<0.01). For reactions associated with injury, all were significant (p<0.01), except for male first time and female first time donors (p>0.05).
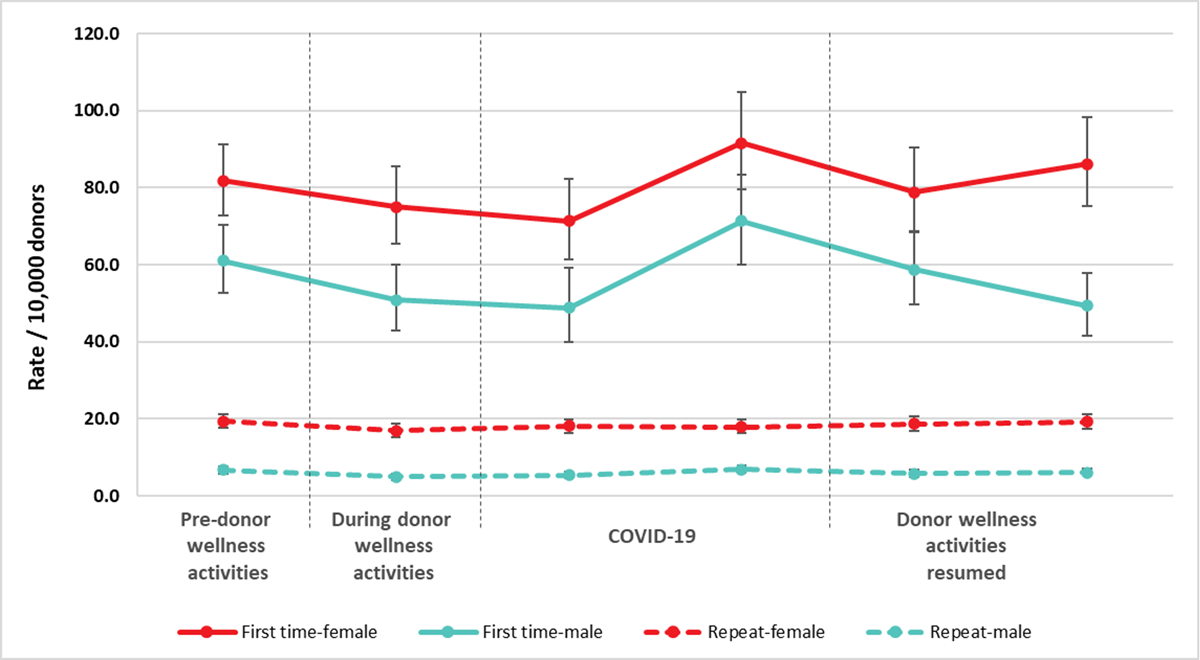
Pre-donation water and salty snacks were put in place in 2019 to reduce vasovagal (faint) reactions. Donor instructions to carry out muscle tension exercises while donating continued to be provided. These can also reduce the risk of a vasovagal reaction. Collectively the pre-donation water and salty snacks, and muscle tension exercises were part of the donor wellness initiative. During the pandemic, collection sites provided post-donation refreshments as always but asked donors to have them outside of the clinic. Collection sites stopped providing salty snacks and water before donating, although donors were encouraged to have their own before coming to the clinic. Between May and June 2022, collection sites increased the distribution of pre-donation salty snack and water. Figure 13 shows the reaction rates before putting in place the donor wellness activities, while they were in place, after stopping the pre-donation water and salty snacks, and during the time donor wellness activities were resumed. Initially there was a downward trend in vasovagal reactions for all groups associated with donor wellness activities. After taking into account gender and donation status, the vasovagal reaction rates were lower during the wellness activities and initially after the water and salty snacks were stopped, but later increased. After the wellness activities resumed, the rates initially returned to the pre-donor wellness level, but then slightly increased for first time females, while first time males continued to decrease (p<0.05). The uptake of wellness activities once resumed may have been less than when first implemented.
Donor hemoglobin and iron
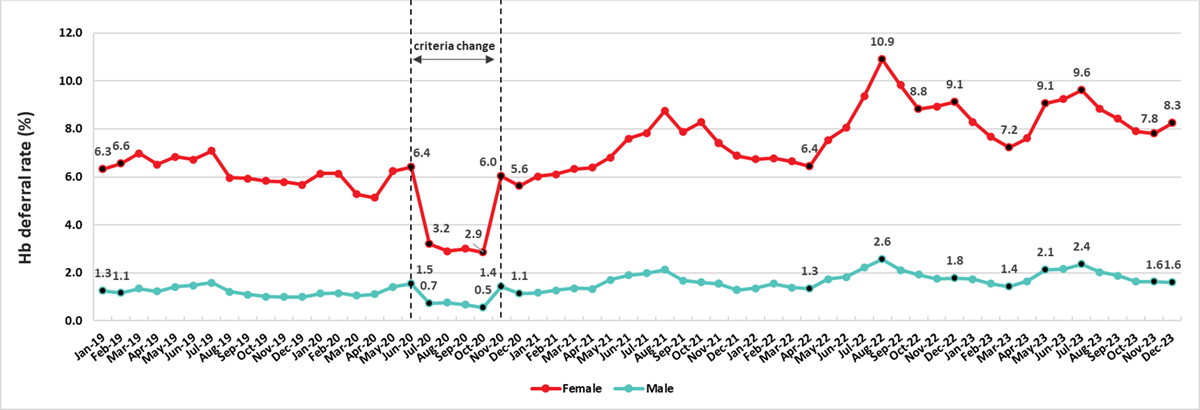
The most common reason for a donor deferral at the collection site is a failed hemoglobin fingerstick screen. Low hemoglobin is often related to low iron stores. Iron is needed to make hemoglobin which carries oxygen in red blood cells. Studies at Canadian Blood Services showed that iron stores are often lower in females and are further reduced by frequent donation in both females and males. Males with borderline hemoglobin are also more likely to have lower iron stores. To reduce the chance of developing iron deficiency, the hemoglobin concentration required for males was increased from 125 g/L to 130 g/L, and the minimum wait time between whole blood donations for females was increased from 56 days to 84 days in 2017. This longer inter-donation period allows females more time to build back their iron stores and return to their baseline hemoglobin levels. To reduce the chance of blood shortages if fewer people donated during the pandemic, the minimum hemoglobin was dropped from July 2020 to October 2020 to 120 g/L for females and 125 g/L for males. As shown in Figure 14 the deferrals decreased from 6.4% to 3.2% (p<0.01) for females and from 1.5% to 0.7% (p<0.01) for males during that period. However, after October 2020, the minimum hemoglobin switched back to the original values and deferrals increased, especially in female donors where they were higher than before the pandemic and remained so. This sustained elevated deferral rate may be related to gradual replacement of hemoglobinometers used to measure hemoglobin with newer equipment, and an increased frequency of donation in repeat donors.
Information about iron and the safety of blood donation can be found at www.blood.ca as well as in the ‘What you must know to give blood’ pamphlet provided to all donors prior to every donation.
Ferritin testing
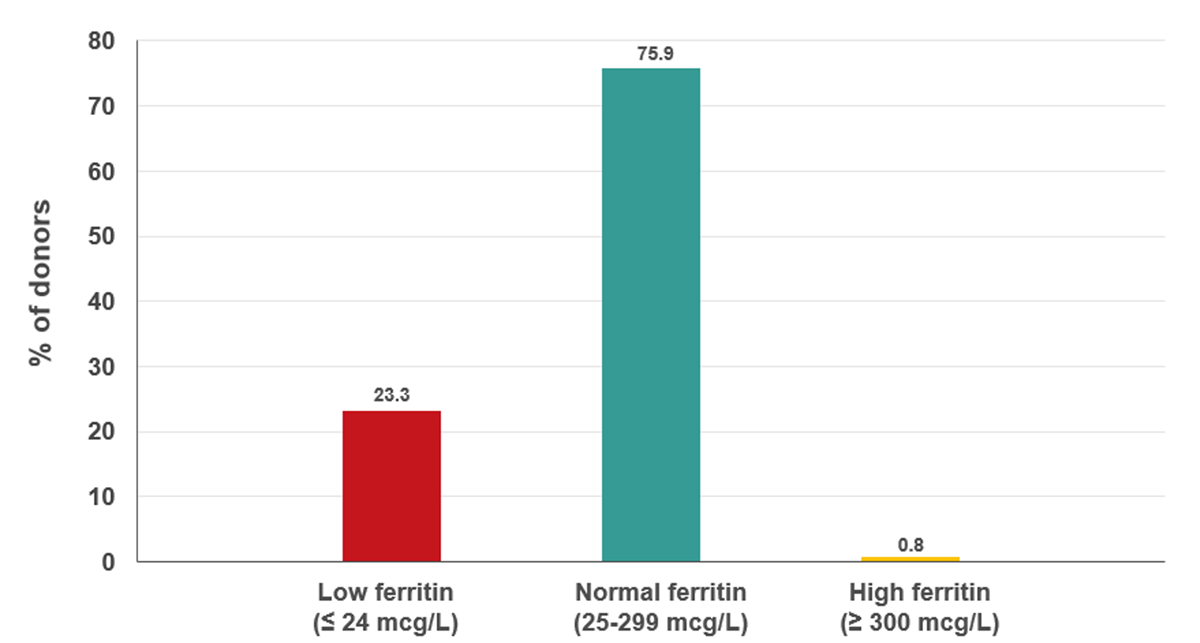
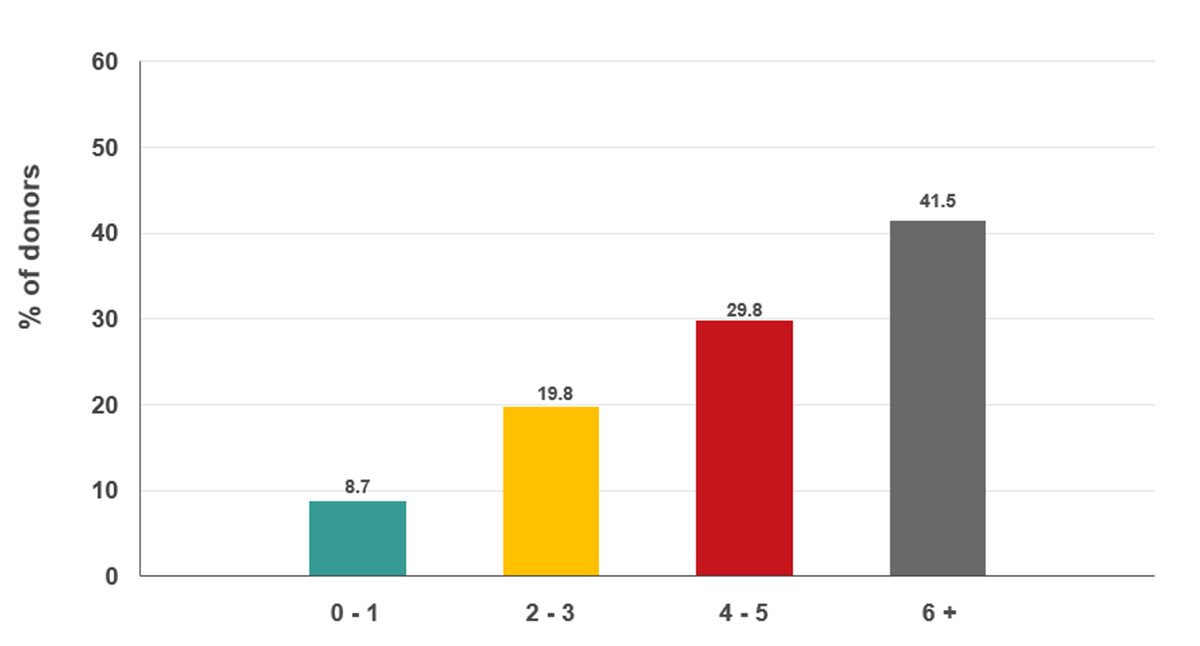
Measuring plasma ferritin can provide an indication of low iron stores before hemoglobin drops. Females are more likely to have low iron stores than males before donating. Iron loss associated with frequent donation is therefore more common in females than males. On Jan. 16, 2023, ferritin testing of every 10th donation in females was implemented. Donors with low ferritin were informed of their result and advised not to donate again for at least six months and to speak to their health care provider about additional testing and possible iron supplementation. As shown in Figure 15, nearly a quarter of females giving their 10th, 20th or 30th etc., donation had low ferritin. Of those who had low ferritin, nearly half had donated six times or more in the previous two years (Figure 16).
Donor demographics
The demographic characteristics of whole blood donors are described in Figures 17 to 20. By region, the distribution of donors ranged from 9.8% in the Atlantic provinces to over 44% in Ontario. The majority of these were repeat donors (79%) and roughly half across all regions were females (49.2%). The demographics of source plasma donors can be found in Section 3.
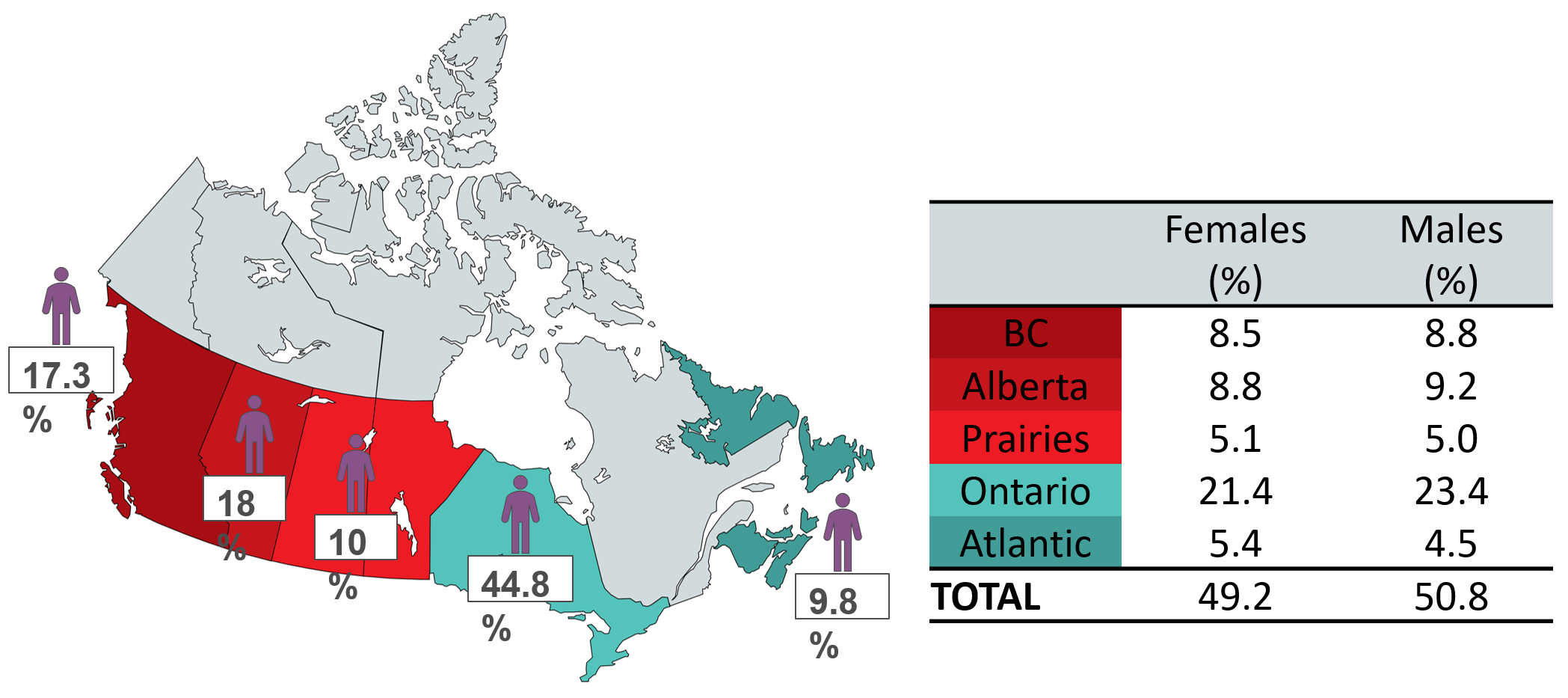
All donors are invited to respond to a voluntary question about their race/ethnic group, which assists the laboratory in selecting donor samples for additional typing for rare blood groups that are more frequent in certain populations. Over 95% of donors in 2022 and 94% in 2023 had answered this question.
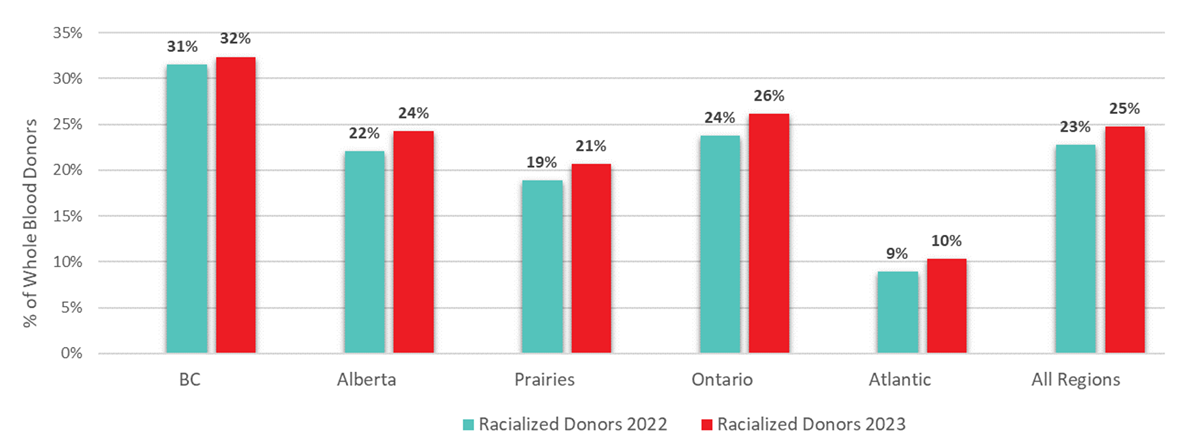
As in previous years, most donations overall were given by donors who self-identify as Caucasian (75.2%). As shown in Figure 18, the proportion of self-described Racialized donors varied significantly by region, ranging from 10% in the Atlantic Region to 32% in British Columbia. The proportion of racialized donors increased slightly in 2023 compared with 2022 across all Canadian regions.
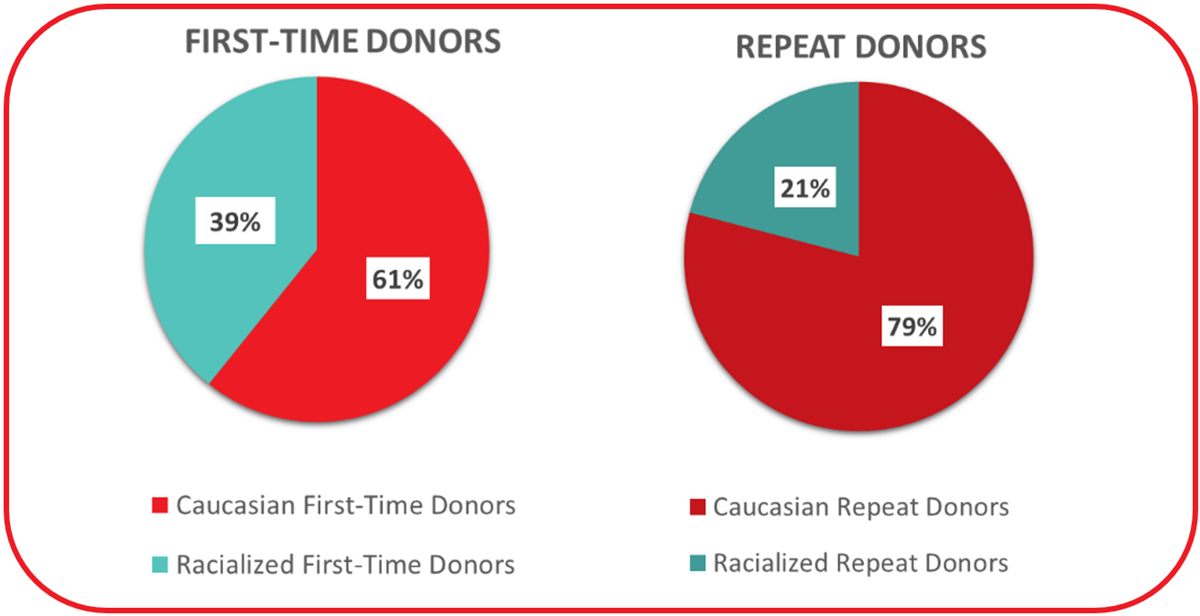
While first time blood donors make up just over 20% of whole blood donors overall, they make up close to 35% of the self-reported Racialized donor group. A higher percentage of first time donors are from Racialized groups (see Figure 19). This is in part related to increasing diversity in younger age groups. In 2023, donors were able to choose from a more comprehensive list to describe their respective backgrounds than in previous years. Of the 24.8% of donors who selected a non-Caucasian background, donors self-identifying as Asian constituted the second largest group of blood donors across all regions (11.5% of all donors providing information) (Figure 20). This was followed by multi-ethnic donors (3.8%) and Filipino donors (2.2%). Arab donors and Indigenous donors (First Nations/Metis/Inuit) constituted 1.6% each of the total donor base, and Black donors 0.75%. Percentages of donors in ethnic groups are similar to their representation in the general population, with the exception of under-representation of Black and Indigenous groups. In the Canadian population approximately 5% are Indigenous and 4.3% Black.
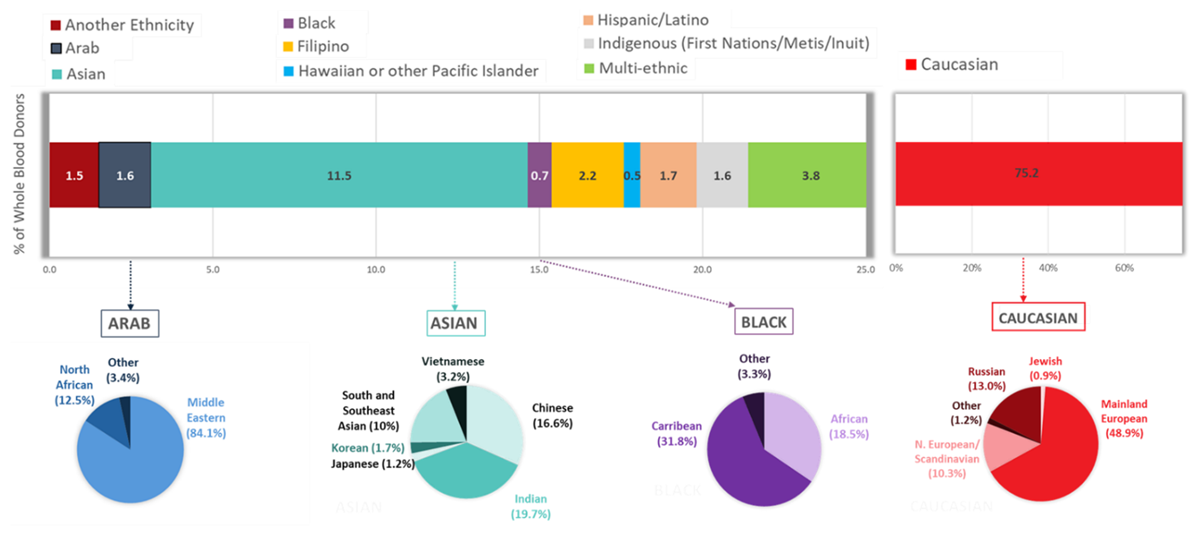
Note: The question for ethnicity/race was modified in 2022 to include more detail, hence some data for the pie charts were missing.
References
Donor screening
Vesnaver E, Goldman M, O’Brien SF MacPherson P, Butler-Foster T, Lapierre D, Otis J, Devine DV, Germain M, Rosser A, et al. Barriers and enablers to source plasma donation by gay, bisexual and other men who have sex with men under revised eligibility criteria: protocol for a multiple stakeholder feasibility study. Health Res Policy Sys 2021;18:131.
O’Brien SF, Goldman M, Robillard P, Osmond L, Myhal G, Roy E. Donor screening question alternatives to men who have sex with men time deferral: Potential impact on donor deferral and discomfort. Transfusion 2021;61:94-101.
Caffrey N, Goldman M, Osmond L, Yi QL, Fan W, O’Brien SF. HIV incidence and compliance with deferral criteria over three progressively shorter time deferrals for men who have sex with men in Canada. Transfusion 2022;62:125-134.
Caffrey N, Goldman M, Lewin A, Osmond L, O’Brien SF. Behaviour-based screening questions and potential donation loss using the “for the assessment of individualized risk” screening criteria: A Canadian perspective. Transfus Med 2022;32:422-427.
Saeed S, Goldman M, Uzicanin S, O’Brien SF. Evaluation of a Pre-exposure Prophylaxis (PrEP)/ Post Exposure Prophylaxis (PEP) Deferral Policy among Blood Donors Transfusion 2021;61:1684-1689.
Germain M, Gregoire Y, Custer BS, Goldman M, et al. An international comparison of HIV prevalence and incidence in blood donor and general population: a BEST Collaborative study. Vox Sang 2021;61:2530-2537.
Goldman M. How do I think about blood donor eligibility criteria for medical conditions? Transfusion 2021;61:2530-2537.
Miller O, Caffrey N, O’Brien SF, Goldman M. Evolving policies for donors with diabetes: The Canadian experience. Vox Sang 2022;117:1415-1419.
Quee F, SF O’Brien, Prinsze et al. Whole blood donor return rates after deferral for tattooing of body piercing-Survey across blood donation services: the BEST collaborative study. Vox Sang 2022;117:1085-1089.
Jacquot C, Tiberghien P, van den Hurk K, Ziman A, Shaz B, Apelseth TO, Goldman M, The BEST Collaborative. Blood donor eligibility for medical conditions: A BEST collaborative study. Vox Sang 2022;117:929-936.
Holloway K, Campbell C, Ali R, et al. A critical contribution in a time of crisis: Examining motivations and deterrents to COVID-19 convalescent plasma donation and future donation intentions among prospective Canadian donors. Transfusion Medicine, 2022;32:351-365.
Etherington C, Palumbo A, Holloway K, et al. Barriers and enablers to and strategies for promoting domestic plasma donation throughout the world: Overarching protocol for three systematic reviews. PLoS ONE 2023;18: e0296104.
Berger, M, Easterbrook, A, Holloway, K, et al. What influences decisions to donate plasma? A rapid review of the literature. Vox Sanguinis, 2023;118: 817-824.
Caffrey N, O’Brien SF, Walsh GM, Haw J, Goldman M. Evolving the gay, bisexual and other men who have sex with men time-based deferral to sexual screening for all donors: The contribution of Canadian research programmes. Vox Sang 2023;118:605-615.
Fisher W A, Kohut T, Woo H, & Haw J. Alternatives to blood donor deferral of gay, bisexual, and other men who have sex with men: Acceptability of screening the sexual risk behavior of all blood donors. Transfusion 2023;63:531–540.
Vesnaver E, Gibson E, Goldman M et al. Navigating imperfect policies to donate plasma: Survey on plasma donation and a pilot plasma donation program among men who have sex with men in Canada. Transfusion 2023;63:1172-1183.
Residual Risk
O’Brien SF, Gregoire Y, Pillonel J, Steele WR, Custer B, Davison K, Germain M, Lewin A, Seed CR. HIV residual risk in Canada under a three-month deferral for men who have sex with men. Vox Sang 2020;115:133-139.
Caffrey N, Goldman M, Lewin A, Gregoire Y, Yi QL, O’Brien SF. Removing the men who have sex with men blood donation deferral: Informing risk models using Canadian public health surveillance data. Transfus Clin Biol 2022;29:198-204.
Babesia microti
O’Brien SF, Drews SJ, Yi QL, Bloch EM, Ogden NH, Koffi JK, Lindsay LR, Gregoire Y, Delage G. Risk of transfusion-transmitted Babesia microti in Canada. Transfusion 2021;61:2958-2968.
Drews SJ, Van Caeseele P, Bullard J, Lindsay LR, Gaziano T, Zeller MP, Lane D, Ndao M, Allen VG, Boggild AK, O’Brien SF, Marko D, Musuka C, Almiski M, Bigham M. Babesia microti in a Canadian blood donor and lookback in a red blood cell recipient. Vox Sang 2022;117:438-441.
Drews SJ, Kjemtrup AM, Krause PJ, Lambert G, Leiby DA, Lewin A, O’Brien SF, Renaud C, Tonnetti L, Bloch EM. Transfusion-transmitted Babesia spp: A changing landscape of epidemiology, regulation, and risk mitigation. J Clin Microbiol 2023;61:e0126822.
Hepatitis E
Fearon MA, O’Brien SF, Delage G, Scalia V, Bernier F, Bigham M, Weger S, Prabhu S, Andonov A. Hepatitis E in Canadian blood donors. Transfusion 2017;57:1420-1425.
Delage G, Fearon M, Gregoire Y, Hogema BM, Custer B, Scalia V, Hawes G, Bernier F, Nguyen ML, Stramer S. Hepatitis E virus infection in blood donors and risks to patients in the United States and Canada. Trans Med Rev 2019;33:139-145.
Hepatitis B
O’Brien SF, Reedman CN, Osiowy C, Bolotin S, Yi QL, Lourenco L, Lewin A, Binka M, Caffrey N, Drews SJ. Hepatitis B blood donor screening data: An under-recognized resource for Canadian public health surveillance. Viruses 2023;15:409
O’Brien SF, Ehsani-Moghaddam B, Goldman M, Drews SJ. Prevalence of hepatitis B in Canadian first time blood donors: Association with social determinants of health. Viruses 2024;16:117
Osiowy C, Giles E, Lowe CF, Matic N, Murphy DG, Uzicanin S, Drews SJ, O’Brien SF. Hepatitis B virus genotype surveillance in Canadian blood donors and a referred patient population, 2016-2021. Vox Sang 2024;119:232-241.
Bacteria
Ramirez-Arcos S, Evand S, McIntyre T, Pang C, Yi QL, DiFranco C, Goldman M. Extension of platelet shelf life with an improved bacterial testing algorithm. Transfusion 2020;60:2918-2928.
Malaria
O’Brien SF, Ward S, Gallian P, Fabra C, Pillonel J, Davison K, Seed CR, Delage G, Steele WR, Leiby DA Malaria blood safety policy in five non-endemic countries: a retrospective comparison through the lens of the ABO risk-based decision-making framework. Blood Transfus 2019;17:94-102.
HTLV
O’Brien SF, Yi QL, Goldman M, Gregoire Y, Delage G. Human T-cell lymphotropic virus: A simulation model to estimate residual risk with universal leukoreduction and testing strategies in Canada. Vox Sang 2018;113:750-759.
Syphilis
O’Brien SF, Drews SJ, Yi QL, Osmond L, Tran V, Zhou HY, Goldman M. Monitoring syphilis serology in blood donors: Is there utility as a surrogate marker of early transfusion transmissible infection behavioral risk? Transfusion 2023;63:1195-1203.
Iron deficiency
Goldman M, Uzicanin S, Osmond L, Yi QL, Scalia V, O’Brien SF. Two year follow-up of donors in a large national study of ferritin testing. Transfusion 2018;25:2868-2873.
Goldman M, Yi QL, Steed T, O’Brien SF. Changes in minimum hemoglobin and interdonation interval: impact on donor hemoglobin and donation frequency. Transfusion 2019;59:1734-1741.
Chasse M, Tinmouth A, Goldman M et al. Evaluating the clinical effect of female donors of child-bearing age on maternal and neonatal outcomes: A cohort study. Transfusion Medicine Reviews, 2020;24:117-123.
Blake JT, O’Brien SF, Goldman M. The impact of ferritin testing on blood availability in Canada. Vox Sang 2022;117:17-26.
Donor wellness
Goldman M, Germain M, Gregoire Y, Vassallo RR, Kamel H, Bravo M, O’Brien SF. Safety of blood donation by individuals over age 70 and their contribution to the blood supply in five developed countries: a BEST Collaborative group study. Transfusion 2019; 59:1267-1272.
Goldman M, Uzicanin S, Marquis-Boyle L, O’Brien SF. Implementation of measures to reduce vasovagal reactions: Donor participation and results. Transfusion 2021;61:1764-1771.
Public health
O’Brien SF, Drews SJ, Lewin A, Russell A, Davison K, Goldman M. How do we decide how representative our donors are for public health surveillance? Transfusion 2022;62:2431-2437.
O’Brien SF, Drews SJ, Lewin A, Osiowy C, Drebot MA, Renaud C. Canadian blood suppliers: An expanded role in public health surveillance? Can Comm Dis Rep 2022;48:124-130.
O’Brien SF, Goldman M, Drews SJ. An expanded role for blood donor emerging pathogen surveillance CMAJ 2023;195:E16.
COVID-19
Stanworth SJ, New HV, Apelseth TO, Goldman M et al. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. The Lancet Haematology 2020;7:757-764.
Drews SJ, Hu Q, Samson R, Abe KT, Rathod B, Colwill K, Gingras AC, Yi QL, O’Brien SF. SARS-CoV-2 virus-like particle neutralization capacity in blood donors depends on serological profile and donor-declared SARS-CoV-2 vaccination history. Microbiol Spectr 2022;10:e0226221.
Lin YJ, Evans DH, Robbins NF, Orjuela G, Hu Q, Samson R, Abe KT, Rathod B, Colwill K, Gingras AC, Tuite A, Yi QL, O’Brien SF, Drews SJ. Utilization of Abbott SARS-CoV-2 IgG II quant assay to identify high-titer anti-SARS-CoV-2 neutralizing plasma against wild-type and variant SARS-CoV-2 viruses. Microbiol Spectr 2022;10:e0281122.
Saeed S, Drews SJ, Pambrun C, Yi QL, Osmond L, O’Brien SF. SARS-CoV-2 seroprevalence among blood donors after the first COVID-19 wave in Canada. Transfusion 2021;61:862-872.
Reedman CN, Drews SJ, Yi QL, Pambrun C, O’Brien SF. Changing patterns of SARS-CoV-2 seroprevalence among Canadian blood donors during the vaccine era. Microbiol Spectr 2022;10:e0033922.
O’Brien SF, Caffrey N, Yi QL, Bolotin S, Janjua NZ, Binka M, Thanh QC, Stein DR, Lang A, Colquhoun A, Pambrun C, Reedman NC, Drews SJ. Cross-Canada variability in blood donor SARS-CoV-2 seroprevalence by social determinants of health. Microbiol Spectr 2023;14:e0335622.
O’Brien SF, Caffrey N, Yi QL, Pambrun C, Drews SJ. SARS-CoV-2 seroprevalence among Canadian blood donors: The advance of Omicron. Viruses 2022;14:2336.
Climate change and infectious risks
Drews SJ, Wendel S, Leiby DA, Tonnetti L, Ushiro-Lumb I, O’Brien SF Climate change and parasitic risk to the blood supply. Transfusion 2023;63:638-645.
Appendix I
Implementation dates for testing
| Marker | Implementation Date* | |
|---|---|---|
| 1 | Syphilis | 1949 |
| 2 | HBV (Hepatitis B Virus) | |
| HBsAG | 1972 | |
| Anti-HBc | 2005 | |
| HBV NAT | 2011 | |
| 3 | HIV (Human Immunodeficiency Virus) | |
| Anti-HIV-1 EIA (enzyme-linked immunosorbent assay | 185 | |
| Anti HIV-1/2 EIA | 1992 | |
| HIV-1 p24 antigen | 1996 (discontinued in 2003, resumed in 2021) | |
| HIV-1 NAT | 2001 | |
| Anti-HIV-1/2 (including HIV-1 subtype O) EIA | 2003 | |
| 4 | HTLV (Human T-Lymphotropic Virus) | |
| Anti-HTLV-I | 1990 | |
| Anti-HTLV-I/II | 1998 | |
| 5 | HCV (Hepatitis C Virus) | |
| Anti-HCV EIA/ELISA | 1990 | |
| HCV NAT | 1999 | |
| 6 | WNV (West Nile Virus) | |
| WNV NAT | 2003 | |
| 7 | Chagas disease (Trypanosoma cruzi | 2010 |
| 8 | Bacteria** | |
| BacT Alert | 2004 | |
| BacT Alert modified after 7 day platelets | 2017 |
*These are the dates that testing for the marker began. Tests have been upgraded as new versions as the test became available.
**Pathogen reduction technology implemented for random donor platelets in 2022/2023.
Appendix II
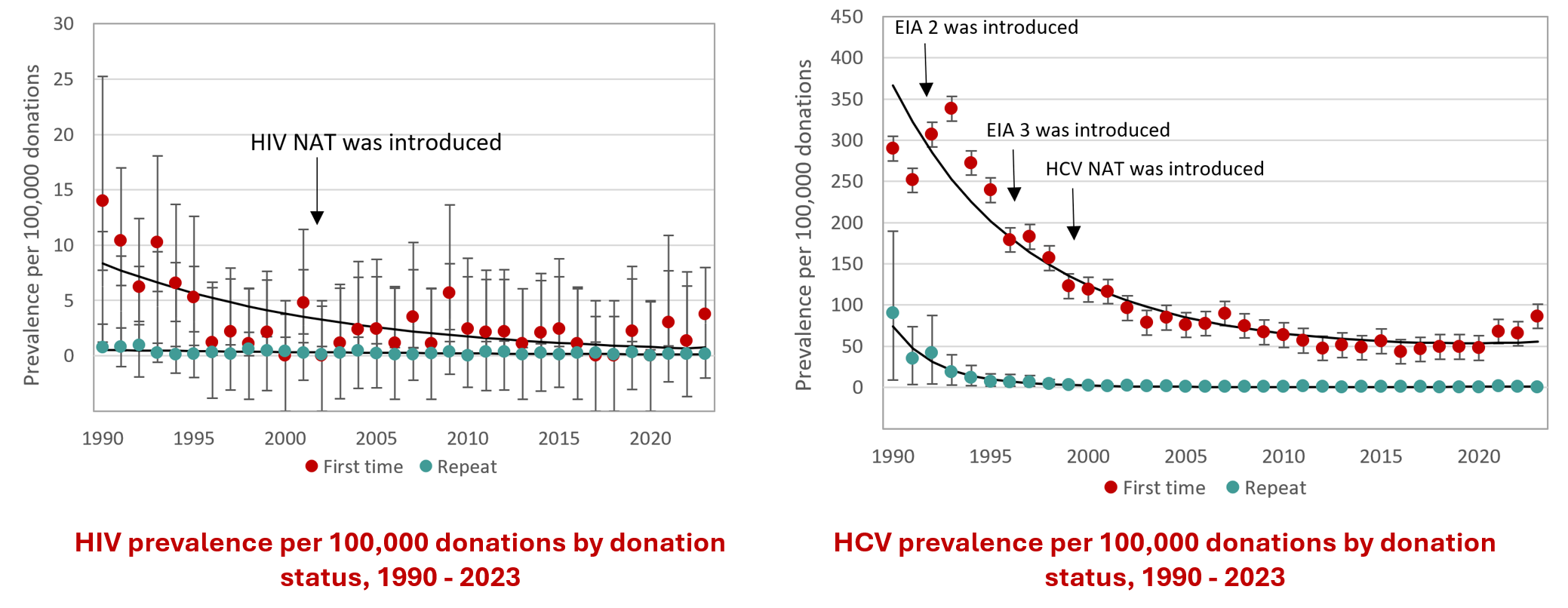
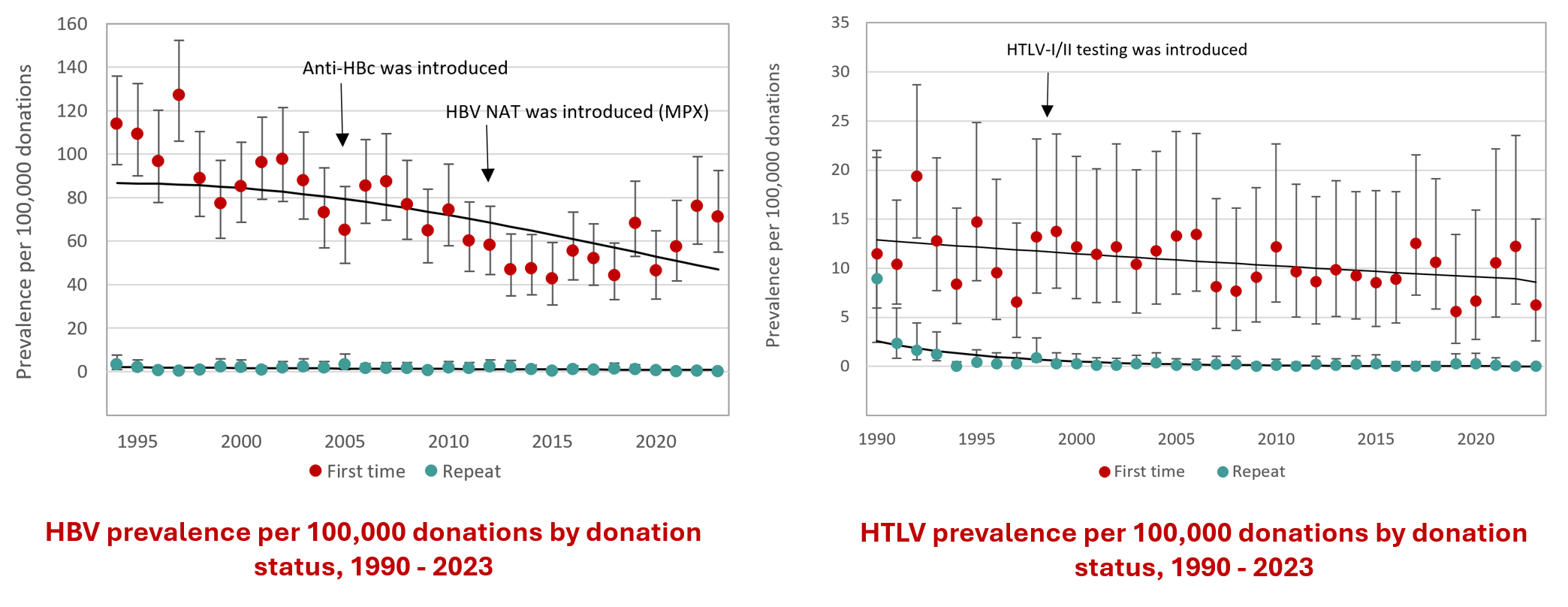
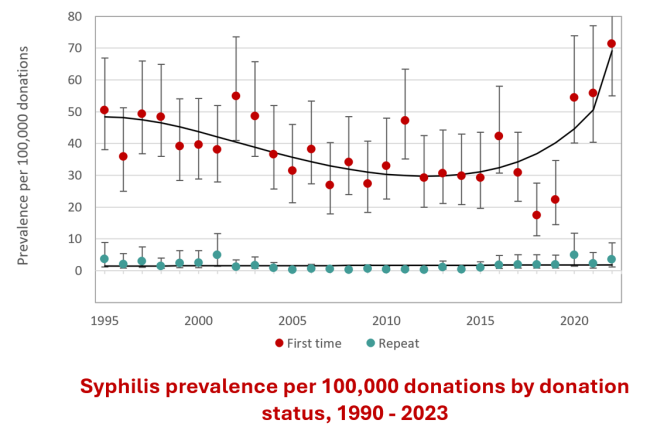
Figure 21: Rate of HIV, HCV, HBV, HTLV and syphilis in first-time and repeat donation. Note that these graphs have different scales on the y-axis.