Introducing pathogen-reduced platelets
What is pathogen inactivation?
Canadian Blood Services’ commitment to blood safety is paramount, which is why many measures are in place to protect transfusion recipients from getting an infection through a blood transfusion. Pathogen inactivation, the latest measure being introduced at Canadian Blood Services, adds an extra layer of protection. By effectively damaging the nucleic acids of a number of pathogens, including viruses and bacteria, pathogen inactivation further reduces the risk of pathogen transmission—an especially important safeguard against new or emerging pathogens, or pathogens for which tests are not available.
In December 2021, Health Canada approved the use of Cerus INTERCEPT™ Pathogen Inactivation Technology to produce pathogen-reduced pooled platelet components at Canadian Blood Services. This approval paved the way for Canadian Blood Services to manufacture its first pathogen-inactivated blood component: pathogen-reduced platelets.
Supported by development work
Years of collaborative teamwork was needed to introduce pathogen-reduced platelets in some Canadian hospitals. Researchers and development scientists at Canadian Blood Services have been evaluating pathogen inactivation technologies, including their potential impact on blood components, for several years. The product and process development group from Innovation and Portfolio Management at Canadian Blood Services led efforts to optimize a process for producing a pooled platelet component that meets the requirements of the INTERCEPT pathogen inactivation system and provide assurance that the treated product would meet quality control requirements. Finally, supply chain tested and validated the manufacturing process in 2021.
Introducing pathogen-reduced platelets at our Ottawa production site
In January 2022, Canadian Blood Services launched pathogen-reduced platelets, also known as pooled platelets psoralen-treated (PPPT). PPPT is now available to hospitals served by our Ottawa production site. Ultimately, Canadian Blood Services intends to make PPPT available at other locations and introduce pathogen-reduced technology for other blood components.
A new resource for health-care professionals
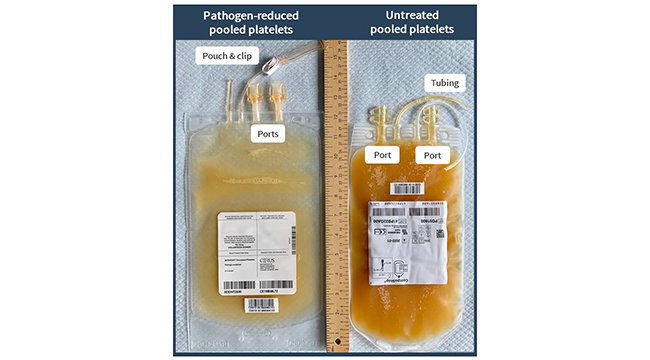
A new resource about PPPT for health care professionals is now available on Canadian Blood Services’ professional education website. The publication, Pathogen-reduced buffy coat platelets, provides information about the technology used to inactivate pathogens, the process of PPPT production, and a comparison of PPPT to untreated platelets, in terms of product characteristics, benefits and drawbacks.
Want to learn more?
- Learn more about pathogen inactivation technologies and how they work in our blog: Pathogen Inactivation - A Primer.
- Learn more about the launch of pathogen-reduced platelets in Canadian Blood Services’ story: Introducing pathogen-reduced platelets to Canada.
- Learn more about the work of the product and process development team in optimizing the process used to manufacture pathogen-reduced platelets in the Centre for Innovation Annual Progress Report 2020-2021 (pages 15-16).
- Read the publication, Pathogen-reduced buffy coat platelets, on our professional education website.
Canadian Blood Services – Driving world-class innovation
Through discovery, development and applied research, Canadian Blood Services drives world-class innovation in blood transfusion, cellular therapy and transplantation—bringing clarity and insight to an increasingly complex healthcare future. Our dedicated research team and extended network of partners engage in exploratory and applied research to create new knowledge, inform and enhance best practices, contribute to the development of new services and technologies, and build capacity through training and collaboration. Find out more about our research impact.
The opinions reflected in this post are those of the author and do not necessarily reflect the opinions of Canadian Blood Services nor do they reflect the views of Health Canada or any other funding agency.